Anatomy of a Clinical Trial
An overview of the clinical trial process, from planning through closeout, this module delves into the design and execution of trials to assess the safety, efficacy, and regulatory compliance of new medicinal products. It covers the key stages of a clinical trial, the roles of various team members, and the essential documents required, ensuring a comprehensive understanding of trial management in line with ICH Good Clinical Practice (GCP) guidelines.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
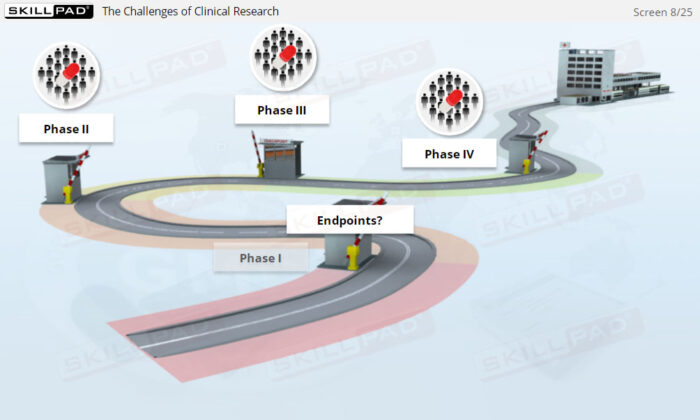
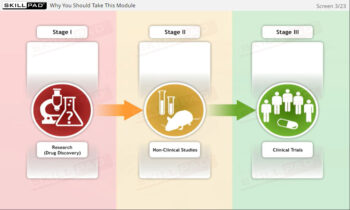
- Distinguish between Clinical Trial Phases: Learn the key stages of a clinical trial—planning, conducting, and closeout—to support effective trial management and regulatory compliance.
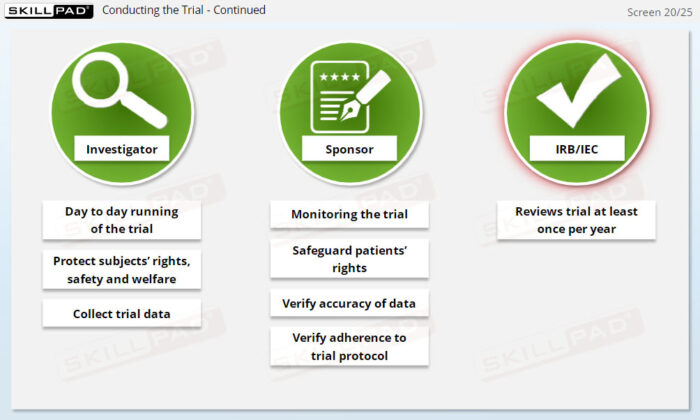
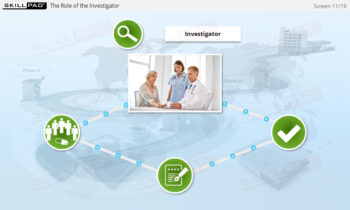
- Recognize Roles and Responsibilities in the Clinical Team: Explore the distinct roles of key team members (e.g., clinicians, biostatisticians, safety representatives) and how they collaborate to ensure successful trial execution.
- Navigate ICH GCP Guidelines: Strengthen your understanding of Good Clinical Practice (GCP) to meet compliance requirements and maintain high ethical and safety standards in clinical research.
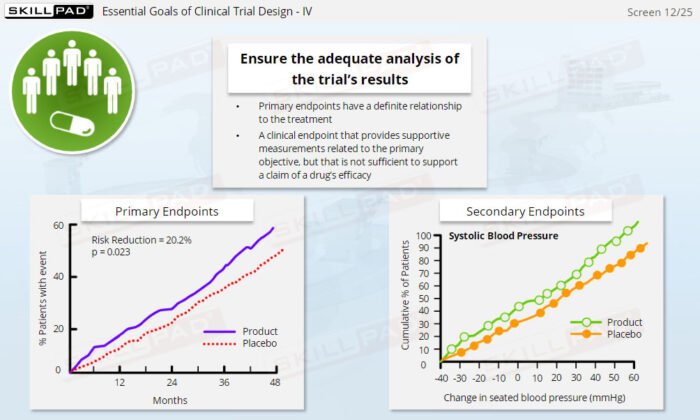
- Work with Essential Clinical Trial Documents: Learn the role of critical documents, such as the clinical trial protocol and case report forms (CRFs), in trial design, data collection, and regulatory submissions.
Learning Objectives
- Understand and explain at an overview level the stages of a clinical trial.
- Describe the roles and responsibilities of the clinical trial team.
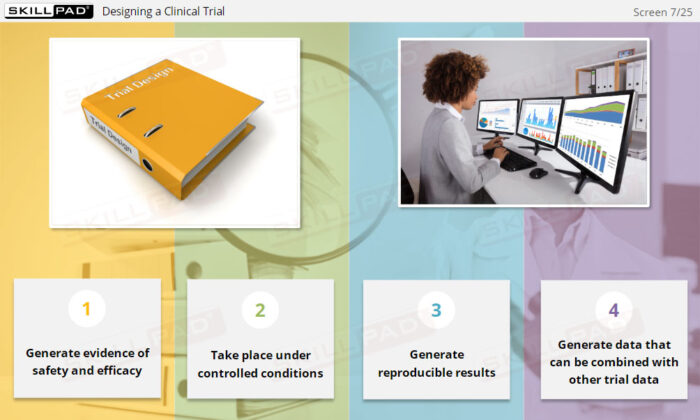
- Explain the key steps involved in planning, designing and conducting clinical trials.
- List the types of documentation in a clinical trial and describe the case reporting form and trial protocol.
- Explain data management in a clinical trial.
Keywords
- Adverse Events
- Biostatistician
- Blinding
- Case Report Forms (CRF)
- Clinical Trial
- Data
- Good Clinical Practice (GCP)
- Informed Consent
- Investigational Product
- Medical Monitor
- Regulatory
- Trial Protocol
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen