API Contamination Prevention
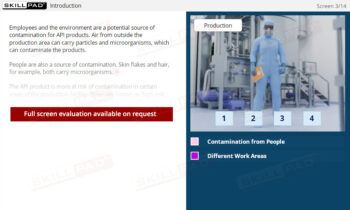
A comprehensive overview of the various types of contamination that can affect Active Pharmaceutical Ingredients (APIs) during production, along with practical steps to minimize these risks. It highlights the importance of personal hygiene, proper use of Personal Protective Equipment (PPE), and effective production practices to help maintain the integrity of APIs. The module also covers the main sources of contamination, including personnel, the environment, and equipment, and offers clear guidelines for preventing contamination and ensuring product safety.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
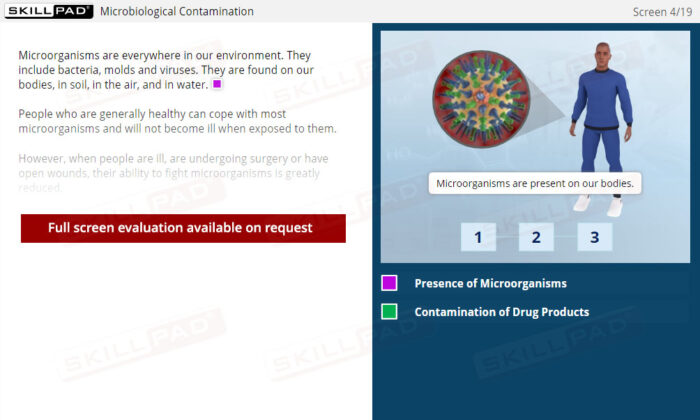
- Understand Contamination Types and Risks: Learn about microbiological, physical, and chemical contaminants that can compromise API quality and patient safety.
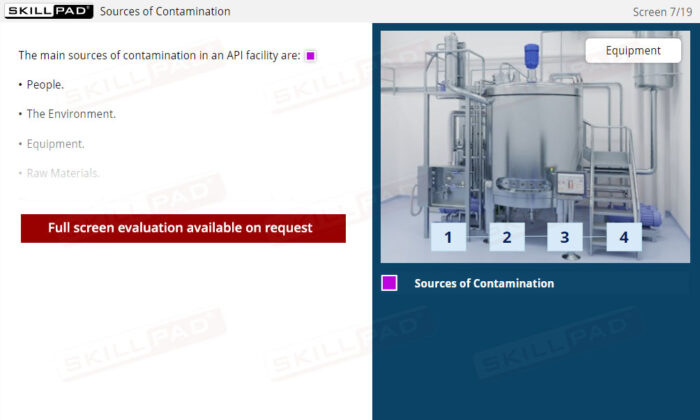
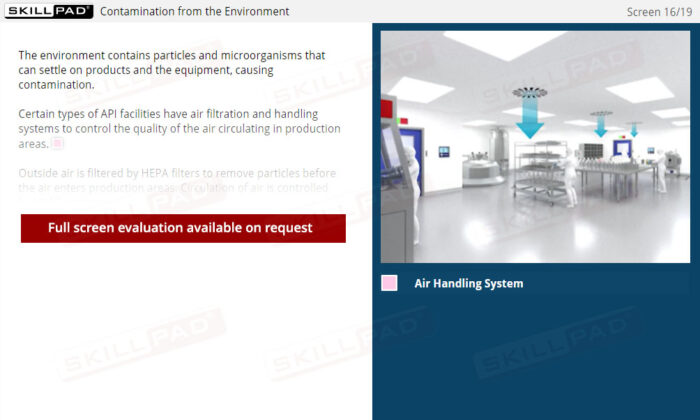
- Reduce Contamination Risks: Identify common sources of contamination in API production, including people, the environment, equipment, and raw materials, and discover best practices to help reduce these risks.
- Use PPE Effectively: Understand the role of Personal Protective Equipment (PPE) in protecting both products and personnel, and learn how to use PPE correctly in different production areas.
- Practice Good Hygiene: Build good habits around personal hygiene, including handwashing, proper attire, and other essential practices to help prevent contamination.
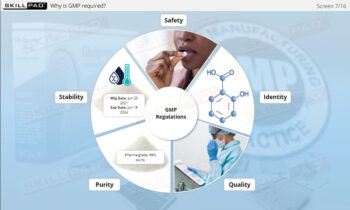
- Gain Safety and Compliance Awareness: Increase awareness of the potential consequences of contamination, supporting the safety of pharmaceutical products and adherence to Good Manufacturing Practices (GMP).
Learning Objectives
- List the different types of contaminants that can be found in an API plant.
- Identify the main sources of contamination in an API plant.
- Describe how to minimize the risk of product contamination.
- Describe the Personal Protective Equipment (PPE) and its purpose.
- List examples of good hygiene and health habits.
Keywords
- Active Pharmaceutical Ingredient (API)
- Contamination
- Contamination Prevention
- Employee Hygiene
- Equipment Cleaning
- Environmental Controls
- Good Manufacturing Practices (GMP)
- Hygiene Practices
- Packaging
- Personal Protective Equipment (PPE)
- Raw Materials
- Supervision
- Training
- Water Quality
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen