Aseptic Processing: Contamination Control
Contamination prevention measures, procedures, and practices that are typically implemented in aseptic processing including the use of cleanrooms, cleanroom gowning, cleanroom cleaning procedures, microbial testing, correct cleanroom behavior, and appropriate personal hygiene.
Part of Annex 1 training requirements.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
- Gain a comprehensive understanding of contamination prevention measures, procedures, and practices in aseptic processing.
- Identify why cleanroom behavior, gowning, cleaning procedures, and microbial testing are crucial for ensuring a safe final product in a cleanroom environment.
- Describe the nature of microorganisms and why they pose a threat to compliance with industry regulations.
Learning Objectives
- Identify the types of microbes that can contaminate cleanrooms and the sources of these microbes.
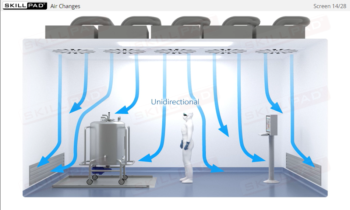
- Outline the key elements of contamination control.
- Describe how correct cleanroom behavior and appropriate personal hygiene contribute to contamination prevention in cleanroom environments.
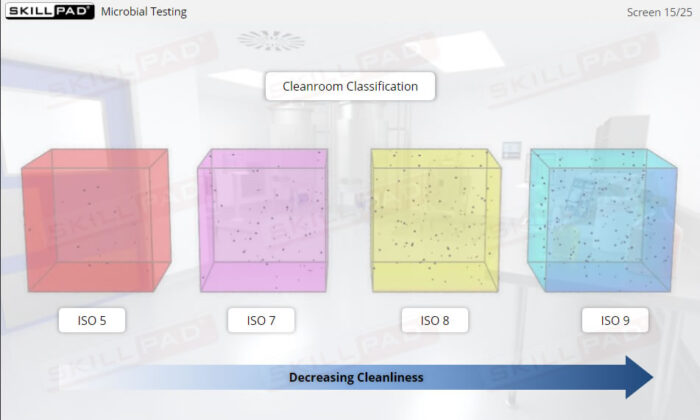
- Explain why gowning requirements change as cleanroom classification becomes progressively stricter.
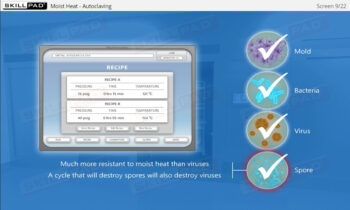
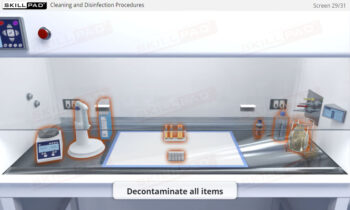
- Describe typical cleaning procedures for cleanrooms.
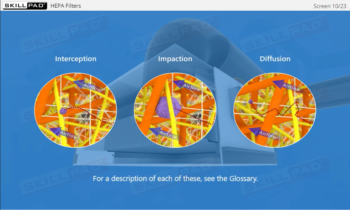
- Describe how microbial testing can be performed in cleanrooms.
Keywords
- Aseptic Processing
- Cleanrooms
- Cleanroom Behavior
- Cleaning Procedures
- Contamination Control
- Gowning, Microbiology
- Microbial Testing
- Personal Hygiene
- Regulatory Compliance
- Quality Assurance
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen