ATMP Manufacturing – Cell and Gene Therapies
This Module is designed for personnel who require an overview of the nature and function of cell and gene therapy products, their modes of manufacture (with a particular focus on autologous and allogeneic therapies), and the associated technical challenges and regulatory requirements.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
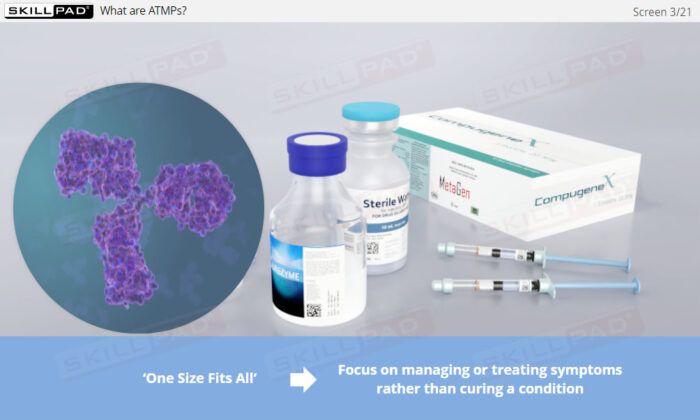
- Gain a clear understanding of Advanced Therapy Medicinal Products, including the differences between cell and gene therapies and how they differ from traditional biologics.
- Learn about the key steps and challenges involved in the manufacturing processes of autologous and allogeneic cell therapies, enhancing your ability to navigate these cutting-edge technologies.
- Understand the hurdles faced in the production of cell and gene therapies, including scale-up limitations, cost concerns, and contamination risks, and explore potential strategies to overcome these challenges.
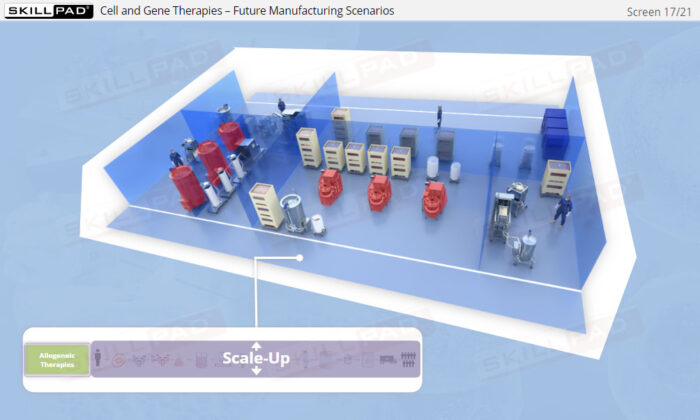
- Explore the future of ATMP manufacturing, focusing on automation and closed processing, and understand how these innovations will shape the scalability and commercial viability of cell and gene therapies.
- Gain insight into the personalized nature of cell and gene therapies, learning how these therapies offer precise treatment options for various diseases, potentially leading to cures rather than mere symptom management.
Learning Objectives
- Define “Advanced Therapy Medicinal Product” (ATMP).
- Explain the difference between ATMPs and ‘traditional’ biopharmaceutical products.
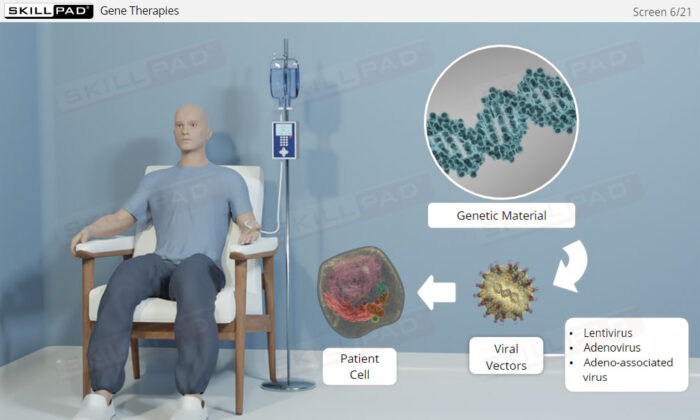
- Explain the difference between gene and cell therapies.
- Describe the biological functionality of cell therapies.
- Describe the biological functionality of gene therapies.
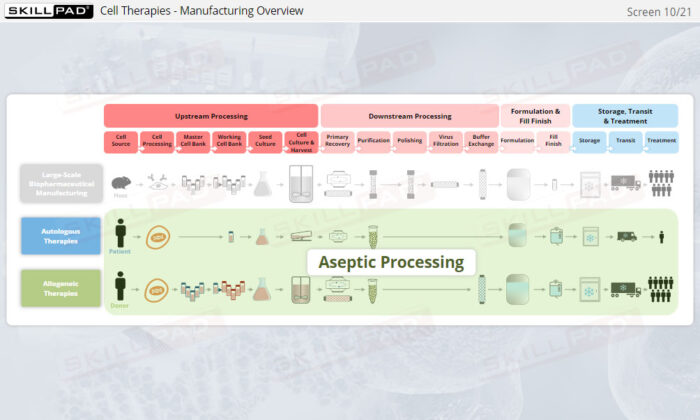
- Distinguish between autologous and allogeneic therapeutic approaches.
- List the main steps in manufacturing autologous therapies.
- List the main steps involved in manufacturing allogeneic therapies.
- Describe the challenges associated with ATMP manufacturing, and in general terms how these challenges might be overcome.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen