Bioprocessing using Single-Use Technologies
This Module is aimed at biopharmaceutical personnel who require a foundational knowledge of single-use systems and how they are used in the manufacture of biopharmaceutical products.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
- Gain a clear understanding of what constitutes single-use technologies and their importance in biopharmaceutical manufacturing.
- Learn the distinctions between disposable laboratory items, simple peripheral items, and equipment for unit operations and platform technologies.
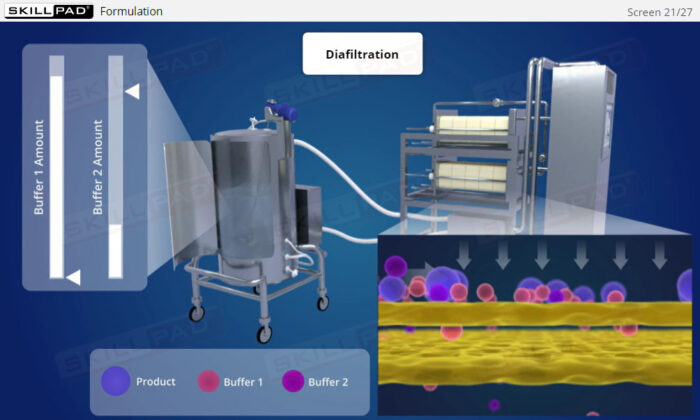
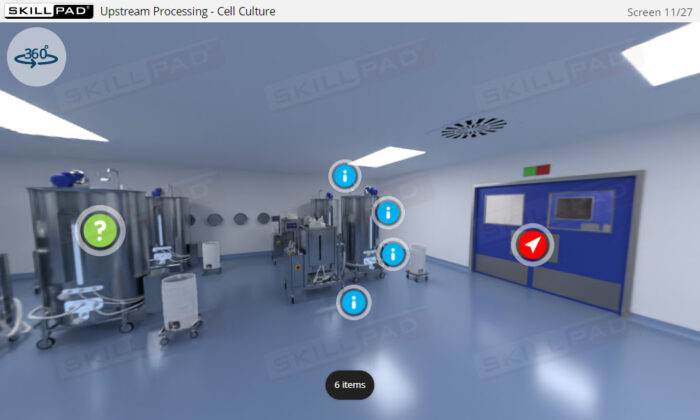
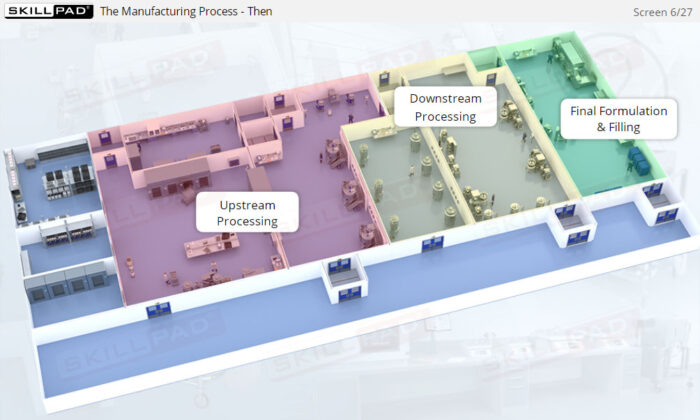
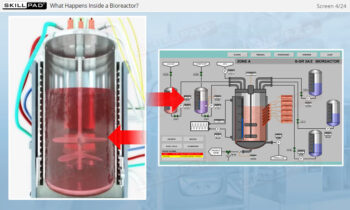
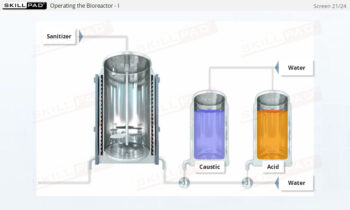
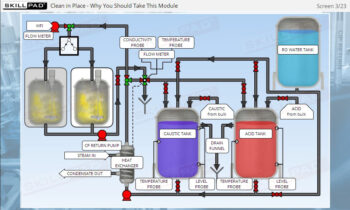
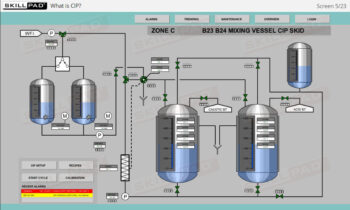
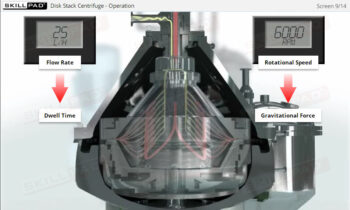
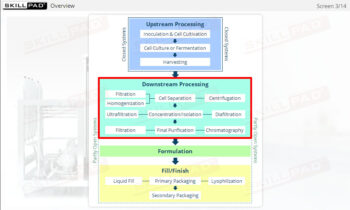
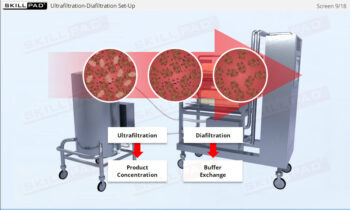
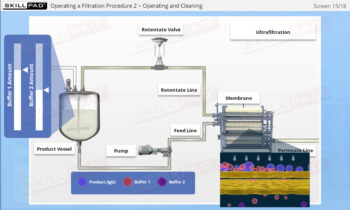
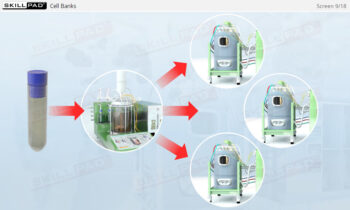
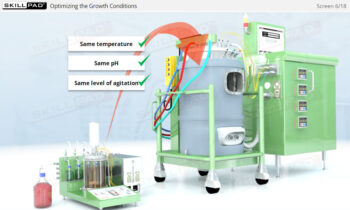
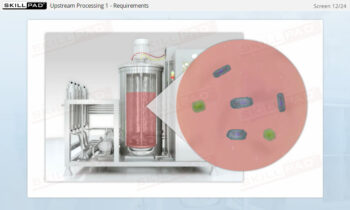
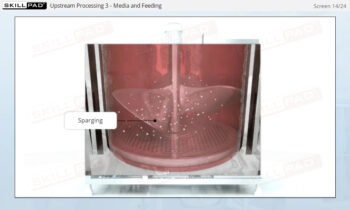
- Discover how SUT is applied in various stages of biopharmaceutical manufacturing, from upstream processing (e.g., pre-culture, cell culture) to downstream operations (e.g., viral filtration, chromatography).
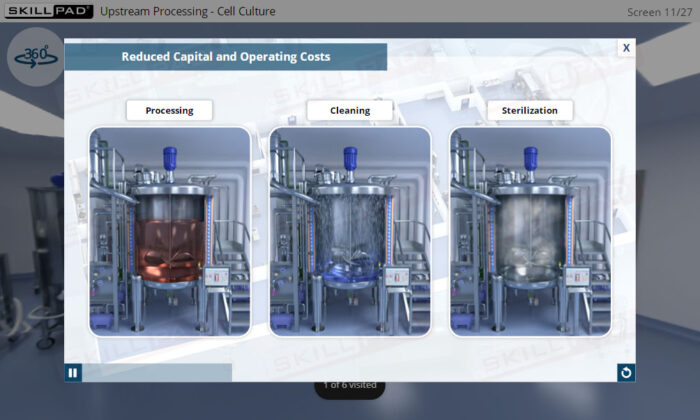
- Appreciate the benefits of SUT, such as reduced cleaning requirements, enhanced flexibility, and increased efficiency in switching between products.
- Recognize the potential challenges and limitations of implementing SUT, including waste management and compatibility issues, to better prepare for practical applications in the field.
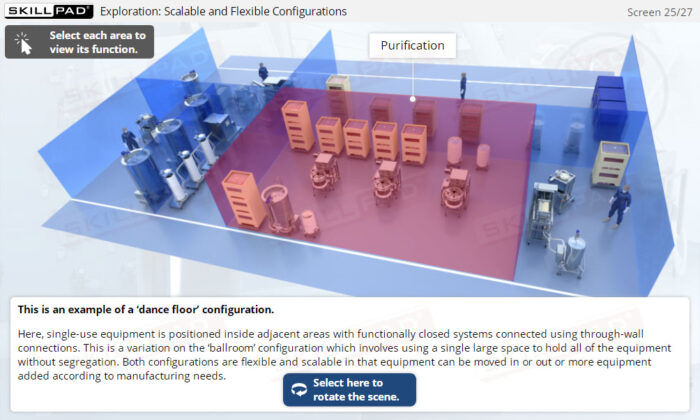
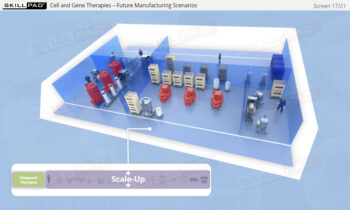
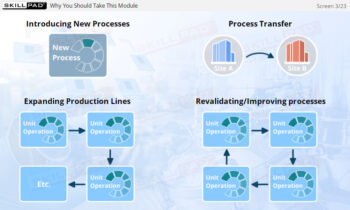
- Understand how SUT facilitates the transition from traditional stainless-steel setups to more flexible, scalable manufacturing processes.
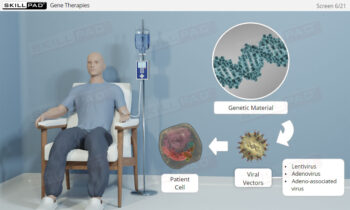
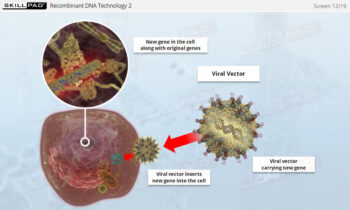
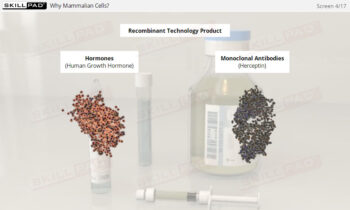
- Learn about the role of SUT in enabling multi-product facilities and supporting the production of next-generation biopharmaceuticals like advanced therapy medicinal products (ATMPs).