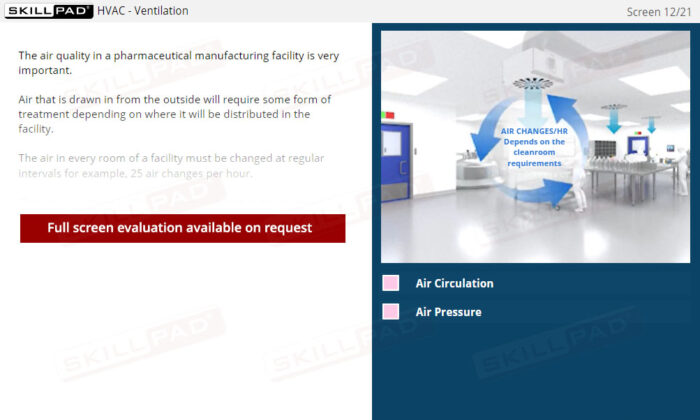
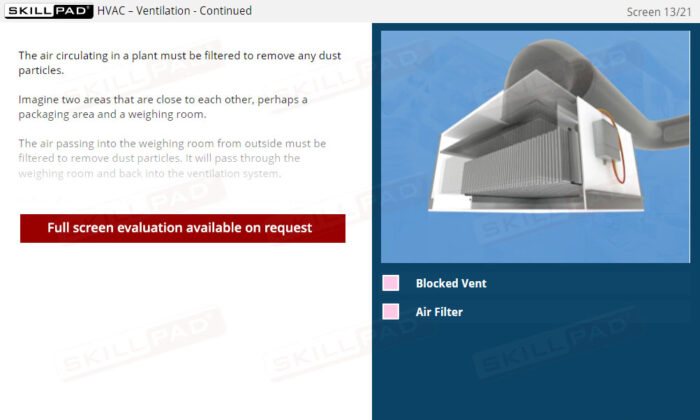
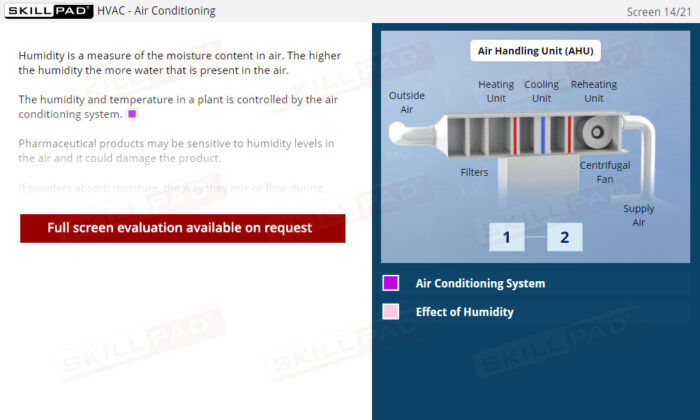
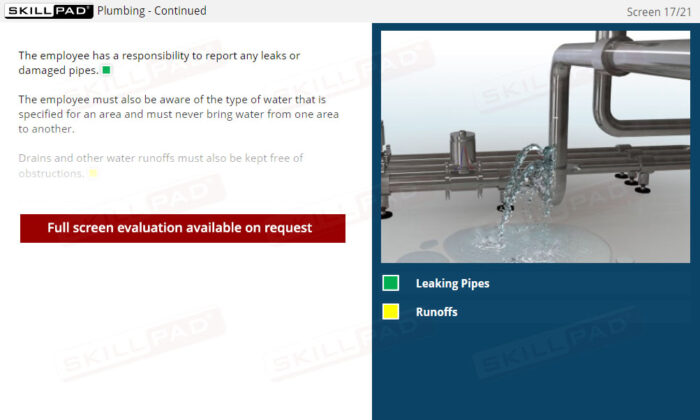
Buildings & Facilities
An overview of the foundational principles of GMP-compliant pharmaceutical plant design, focusing on minimizing contamination risk through strategic facility layout, effective product flow, and robust environmental control systems, including HVAC and plumbing. This module also covers critical practices in cleaning, sanitation, and maintenance that uphold the highest standards of product quality and safety.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
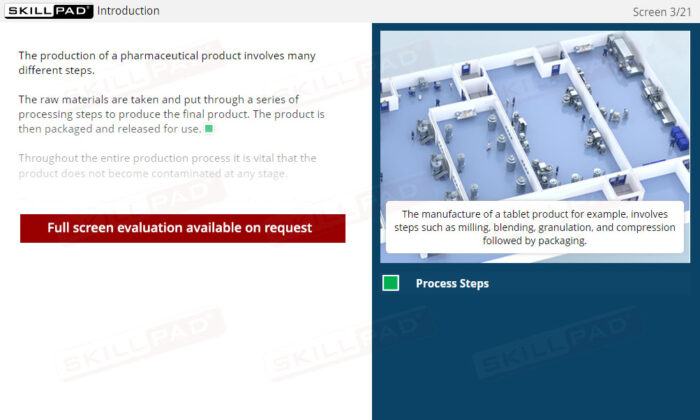
- Learn About GMP Facility Design: Understand how internal and external facility layouts are strategically constructed to reduce contamination risks and support efficient manufacturing processes.
- Understand Product Flow Management: Learn the importance of product flow in preventing cross-contamination, including the use of segregated production areas and logical sequencing of manufacturing steps.
- Learn About Environmental Controls: Understand the role of HVAC systems in regulating temperature, humidity, and air quality, and the measures used to maintain a controlled environment for sensitive pharmaceutical products.
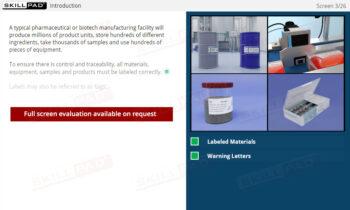
- Recognize the Importance of Plumbing and Water Quality: Gain awareness of the significance of a well-designed and maintained plumbing system in contamination prevention, including proper water management to avoid stagnant water.
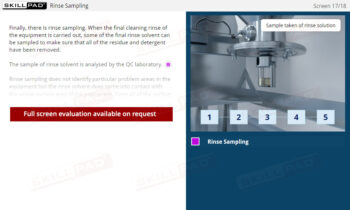
- Follow Sanitation and Maintenance Protocols: Learn sanitation protocols and understand each employee’s role in maintaining clean, operational facilities, including when and how to report issues requiring expert attention.
Learning Objectives
- Explain how pharmaceutical plants are designed.
- Describe the concept of product flow.
- List and explain the environmental controls employed in pharmaceutical plants.
- Describe why a proper plumbing system is necessary.
- Explain the importance of good cleaning, sanitation, and maintenance procedures.
Keywords
- Air Quality
- Cleanroom Standards
- Contamination Prevention
- Cross-Contamination
- Environmental Controls
- Good Manufacturing Practices (GMP)
- Humidity
- HVAC System
- Product Flow
- Temperature
- Water Quality
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen