Cell Culture in Biopharmaceutical Manufacturing
This Module is aimed at biopharmaceutical personnel who require a foundation knowledge of cell culture as part of upstream processing in biopharmaceutical manufacturing. It describes mammalian cell culture in the biopharmaceutical industry, how such cultures are controlled and important considerations in maintaining optimal cultures.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
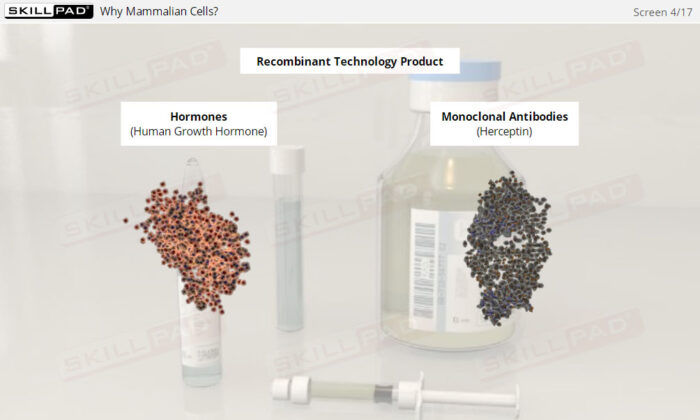
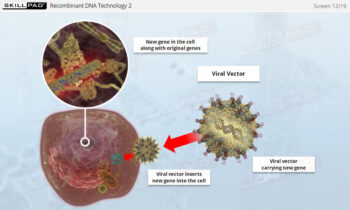
- Gain insights into why mammalian cells are favored in producing complex therapeutic proteins, including the necessity for post-translational modifications that only mammalian cells can perform.
- Learn about the complex nutritional needs of mammalian cells, including amino acids, vitamins, and growth factors, and how these are met in a controlled environment.
- Distinguish between suspended and adherent cell cultures, as well as batch and continuous culture systems.
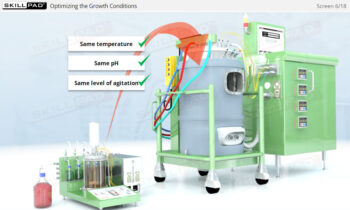
- Enhance your technical skills by learning to monitor and control essential parameters like temperature, pH, and dissolved oxygen to ensure optimal cell growth and product quality.
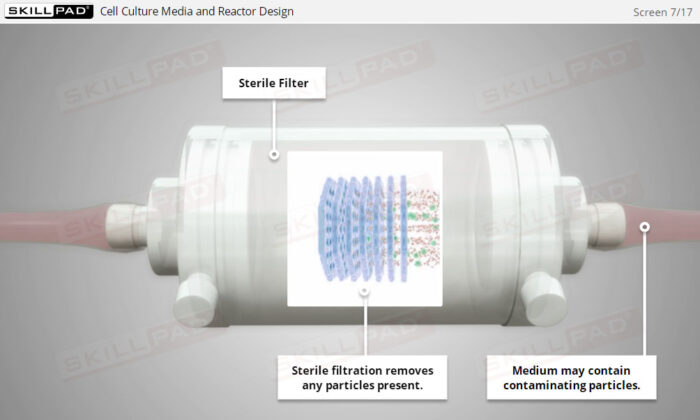
- Understand the importance of sterility in cell culture, including sterilization techniques and the potential impacts of contamination on biopharmaceutical production.
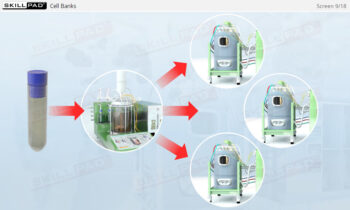
- Equip yourself with knowledge about the critical roles of cell banks in maintaining cell line consistency and quality, as well as the procedures for inoculating and scaling up cultures.
- Learn how to perform cell counting using a hemocytometer, including techniques to distinguish live cells from dead ones, ensuring accurate quantification for successful cell culture operations.
Learning Objectives
- Describe the advantages of using mammalian cells in the manufacture of protein-based therapeutic products.
- Describe the typical composition of growth media used to culture mammalian cells.
- Explain the concept of a cell bank, and how a typical inoculation procedure is performed.
- Distinguish between suspended and adherent cell culture.
- List the process parameters that are typically monitored and controlled in a cell culture process.
- Distinguish between batch and continuous cell culture.
- Explain how microbial contamination can adversely affect a cell culture process.
- Explain the purpose of cell counting, and how it can be performed.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen