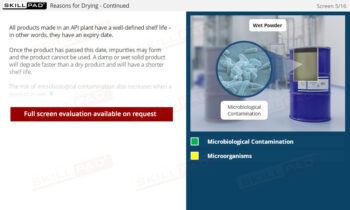
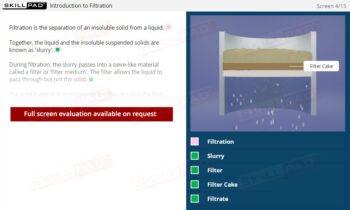
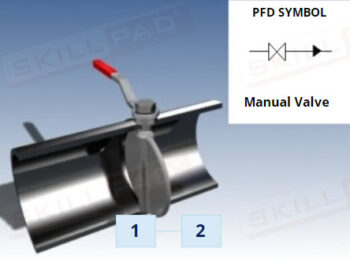
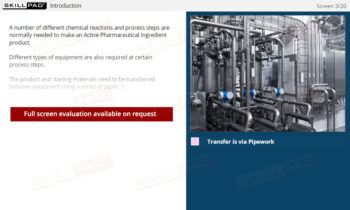
Chemical Reactions: Overview
An overview of chemical reactions in API (Active Pharmaceutical Ingredient) manufacturing, with a focus on controlling key process variables to help ensure high-quality products. It covers the importance of managing chemical reactions to minimize impurities, maintain yield, and follow precise procedures outlined in Batch Production Records. Learners will explore the roles of starting materials, intermediates, solvents, and process variables such as temperature, mixing speed, and reaction time in supporting consistent and safe pharmaceutical production.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand Chemical Reactions in API Manufacturing: Learn the fundamentals of chemical reactions, including the role of starting materials and the formation of intermediates, and how these contribute to producing high-quality API products.
- Manage Impurity Control: Discover how to identify and reduce impurities in chemical reactions to support the purity and safety of the final product and protect patient health.
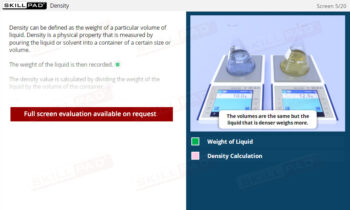
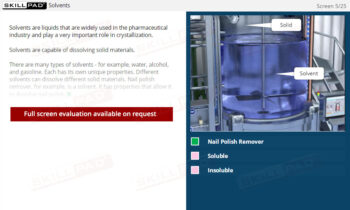
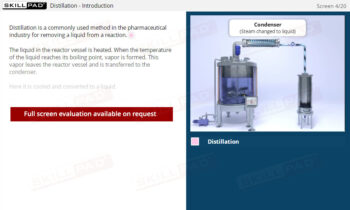
- Control Process Variables: Gain knowledge of key process variables such as ingredient weight, solvent volume, temperature, and mixing time, and understand how controlling these factors helps optimize reaction outcomes and product yield.
- Recognize the Importance of Batch Production Records (BPRs): Understand how Batch Production Records help track and verify the consistency of each step in the chemical reaction process, ensuring compliance and identifying potential issues early.
- Build Operational Awareness: Learn how changes in the reaction process, such as variations in solvent volume or mixing speed, can affect product yield and purity, and explore ways to address these variations to help maintain product quality.
Learning Objectives
- Explain why chemical reactions must be controlled.
- Distinguish between starting materials and intermediate materials.
- Explain why impurities must be controlled in a chemical reaction.
- Explain what is meant by yield in a chemical reaction.
- List the common process variables in a chemical reaction.
- Recognize the importance of following batch production records.
Keywords
- Active Pharmaceutical Ingredient (API)
- Agitator
- Batch Production Records (BPR)
- Chemical Reactions
- Cooling
- Exothermic
- Impurities
- Intermediate
- Mixing Time
- Process Variables
- Reaction Time
- Solvent Volume
- Starting Materials
- Temperature
- Yield
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen