Clean in Place
Explains key concepts of Clean In Place (CIP) technology commonly used in the biotechnology and pharmaceutical industries. It describes CIP processes and procedures and provides examples of best practices that help ensure optimum performance.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
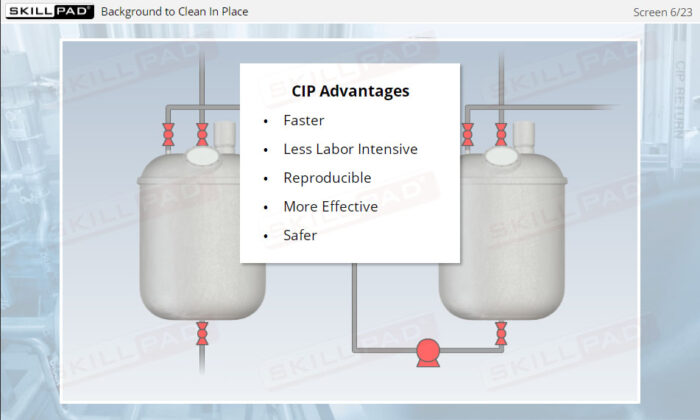
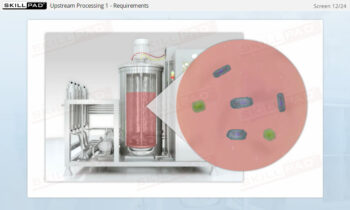
- Gain a foundational understanding of Clean In Place technology and its critical role in maintaining sterile and efficient manufacturing environments in the pharmaceutical and biologics industries.
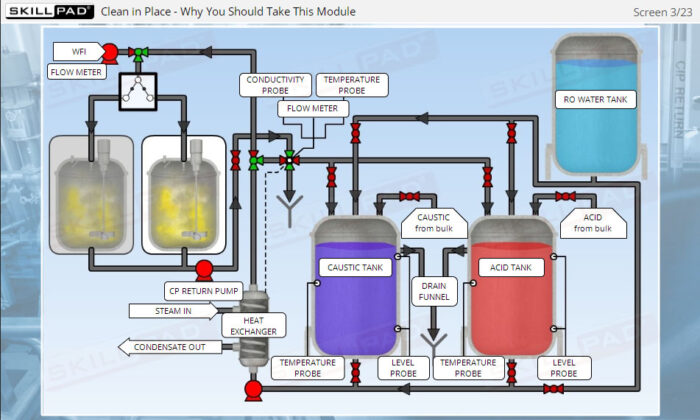
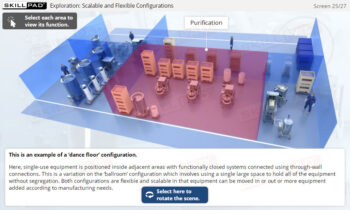
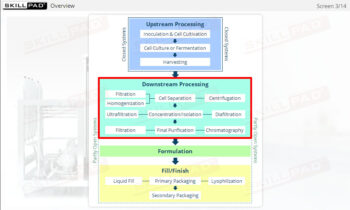
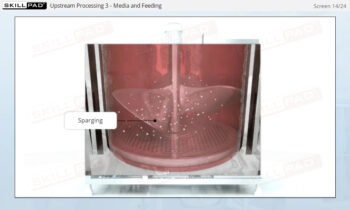
- Learn about the various stages of a CIP
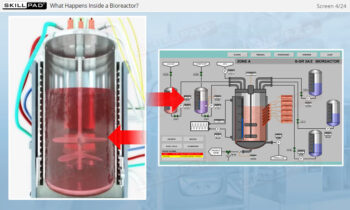
operation, the typical components of a CIP system, and how these elements work together to ensure effective cleaning.
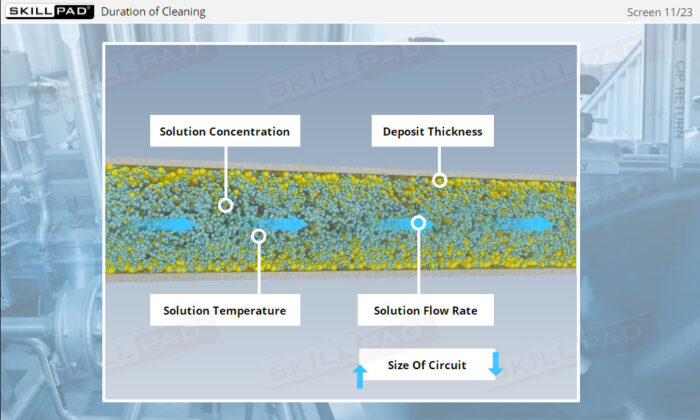
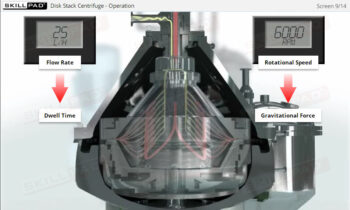
- Identify the main variables affecting CIP performance and learn how to troubleshoot common issues, allowing you to optimize cleaning operations and minimize downtime.
- Appreciate the importance of consistent and effective CIP processes in preventing contamination and ensuring compliance with industry standards and regulations.
- Recognize the potential safety and environmental hazards associated with CIP operations and the necessary precautions to reduce these risks.
- Learn to ensure and verify successful cleaning processes, thus maintaining high product quality and preventing costly production issues related to contamination or equipment failure.
Learning Objectives
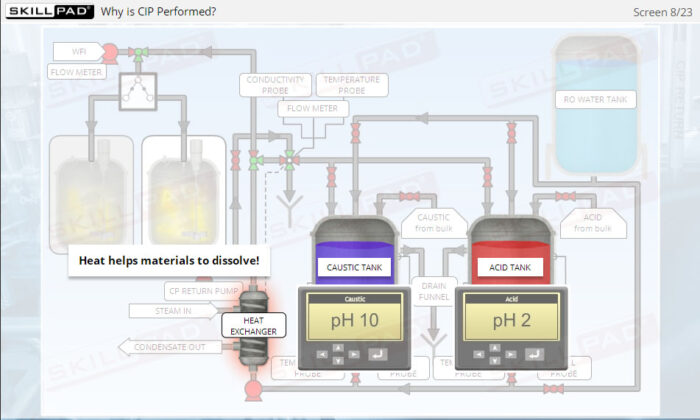
- Explain the importance of CIP in manufacturing.
- Describe why and when a CIP operation should be performed.
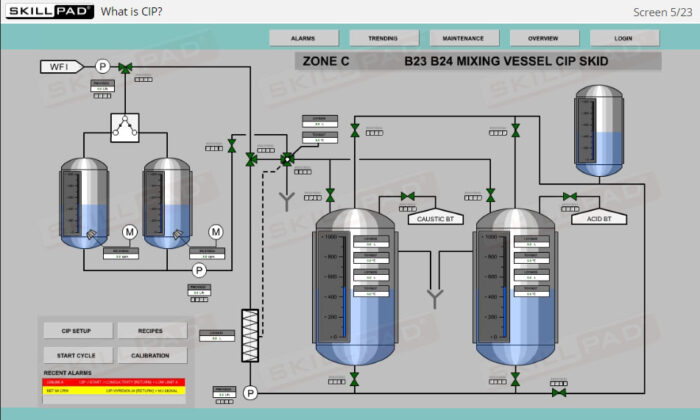
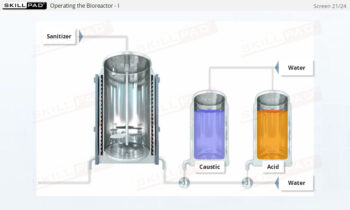
- List the typical components of a CIP system.
- Explain the stages of a CIP operation.
- Explain the main variables that affect the performance of a CIP operation.
- Explain common CIP troubleshooting steps and explain where the CIP process can go wrong.
- List the critical actions that you should take to ensure a CIP operation runs as efficiently as possible.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen