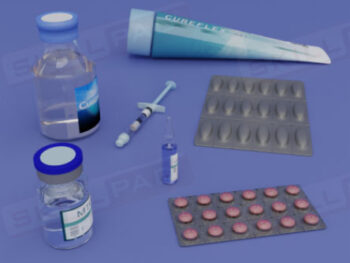
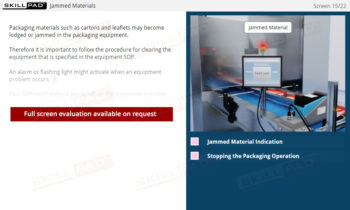
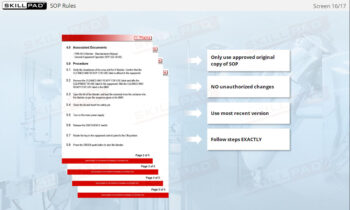
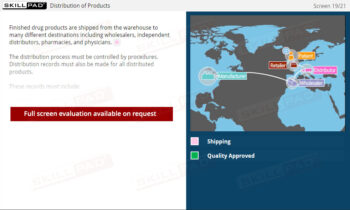
Cleaning of Equipment
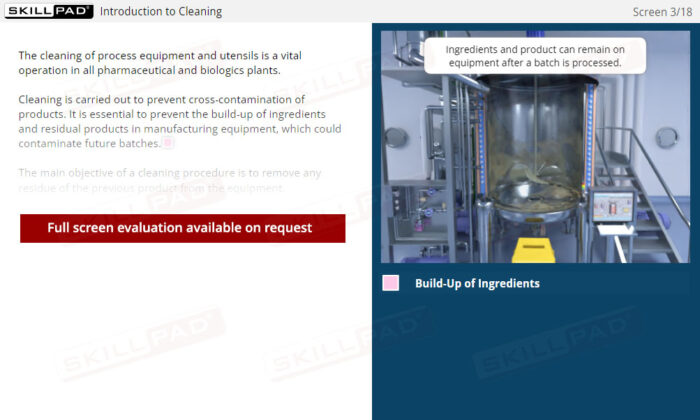
An overview of essential cleaning practices for equipment in pharmaceutical and biologics manufacturing. This module explains the importance of preventing cross-contamination, outlines the two main categories of equipment— dedicated and non-dedicated—and introduces different cleaning methods, including Manual, Clean In Place (CIP), and Clean Out of Place (COP). Learners will gain insights into the requirements for cleaning Standard Operating Procedures (SOPs) and techniques for verifying equipment cleanliness, such as direct surface and rinse sampling.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Learn About Equipment Cleanliness: Understand why thorough equipment cleaning is critical to avoid cross-contamination and ensure patient safety, with an emphasis on GMP compliance.
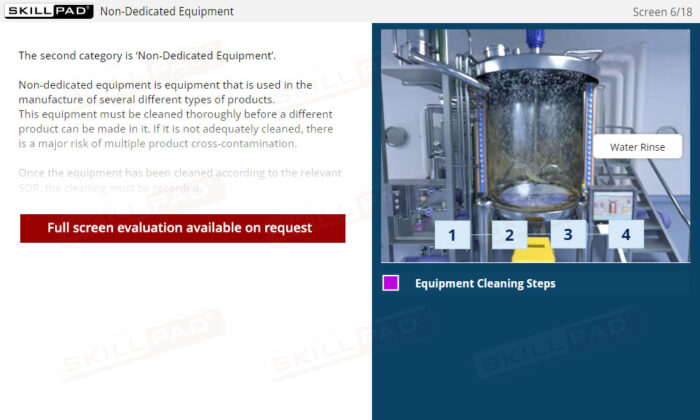
- Understand Equipment Categories: Learn the differences between dedicated and non-dedicated equipment and how cleaning requirements vary to support safe production practices.
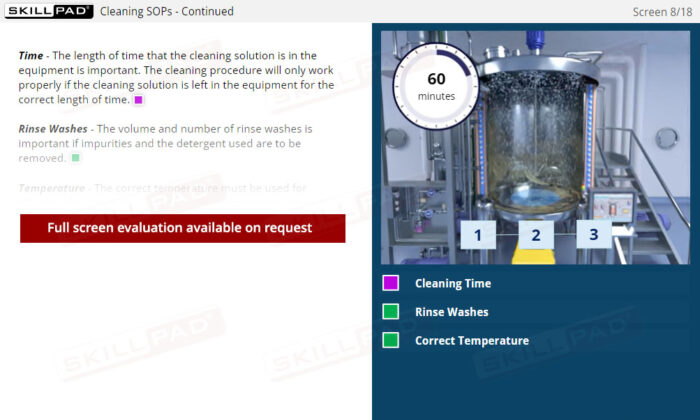
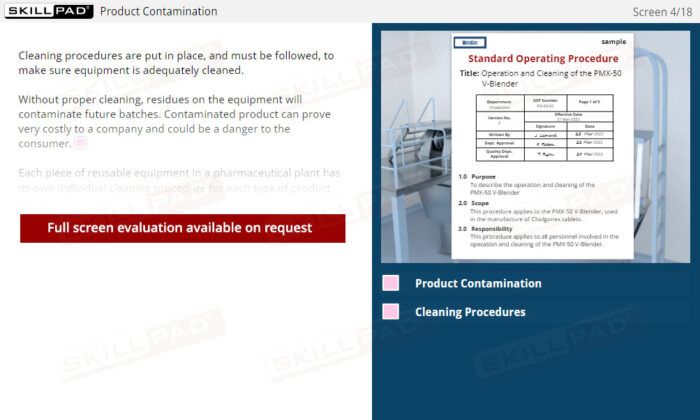
- Learn About Cleaning SOPs: Discover the key components of a cleaning SOP, such as detergent concentration and temperature, and why each element is important for effective cleaning.
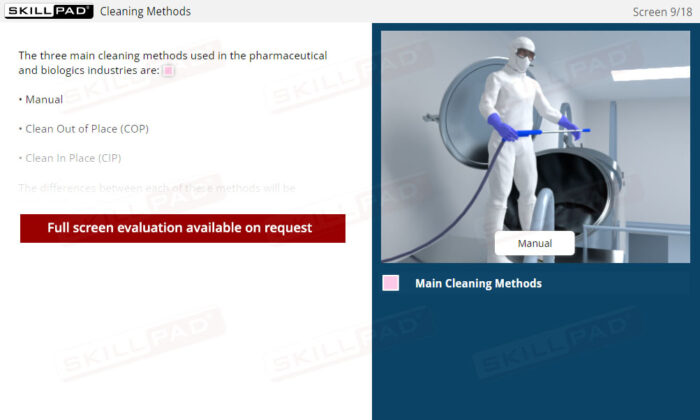
- Explore Cleaning Methods: Understand the advantages and limitations of manual cleaning, CIP, and COP to make informed decisions about cleaning processes and efficiency.
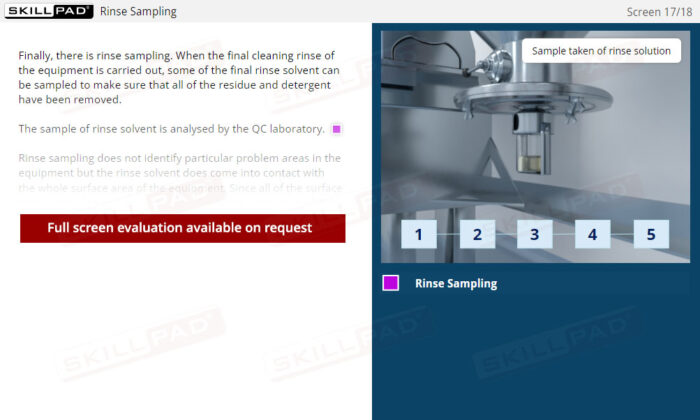
- Understand Cleaning Verification: Learn about direct surface sampling and rinse sampling techniques to confirm equipment cleanliness and ensure product quality and understand how to avoid sampling errors.