Contamination Control Strategy – Annex 1 and QRM
Explains the ‘why’ behind Contamination Control Strategy (CCS) and Quality Risk Management (QRM) for all employees involved in sterile product manufacture. It provides a solid understanding of CCS and the holistic nature of contamination control, ultimately improving CCS design and implementation.
Interested in the ‘how’ of QRM and CCS? Please see our companion module Contamination Control Strategy – QRM in Practice!
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
- Gain a thorough understanding of the EU GMP Annex 1 guideline and its purpose in sterile product manufacturing.
- Gain practical knowledge on devising, implementing, and maintaining a Contamination Control Strategy (CCS) in accordance with Annex 1 requirements.
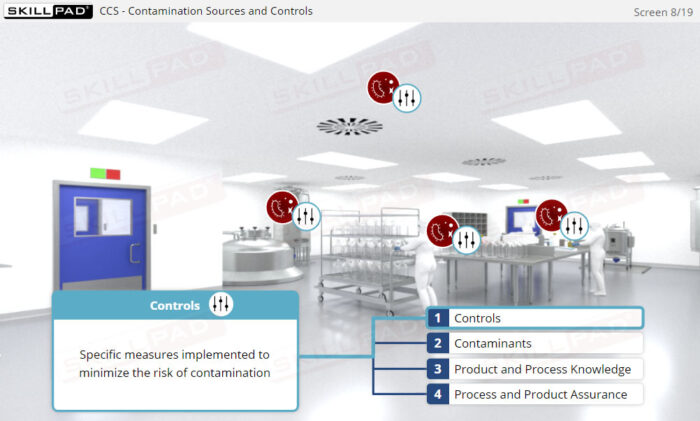
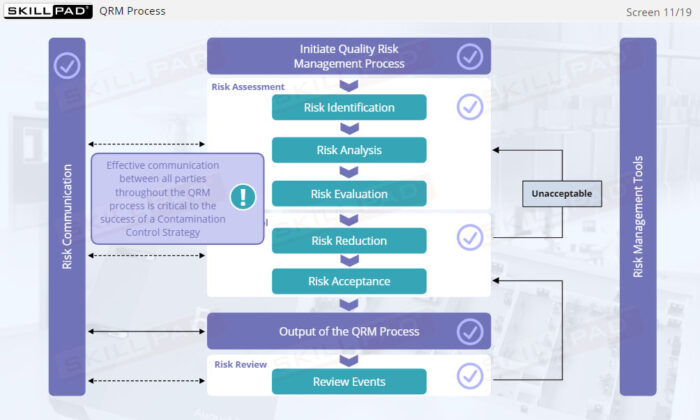
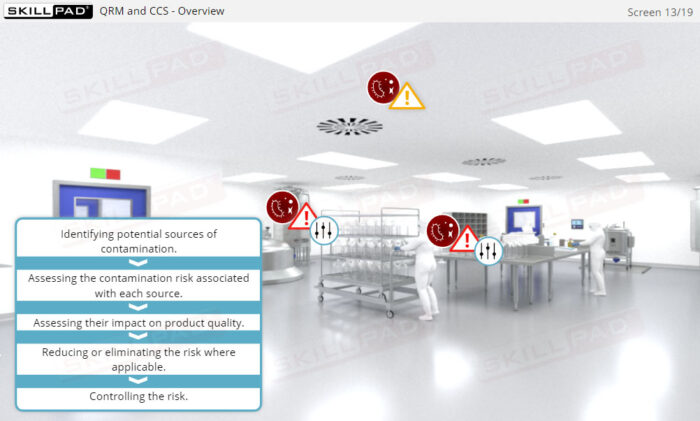
- Learn the concept of Quality Risk Management (QRM) and its application to CCS development, including the four steps of the QRM process: risk assessment, risk control, risk communication, and risk review.
- Acquire problem-solving skills to identify Critical Control Points (CCPs) and understand their role in CCS implementation, as well as the importance of reviewing and updating the CCS for continuous improvement in contamination control.
Learning Objectives
- Explain the purpose of the EU GMP Annex 1 guideline.
- Define Contamination Control Strategy (CCS) as per Annex 1.
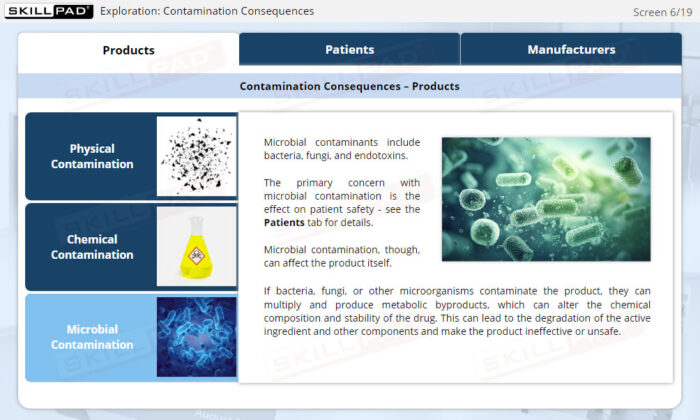
- Describe the potential consequences of contamination in sterile product manufacturing.
- Explain the concept of Quality Risk Management (QRM) and its application to CCS development.
- Describe the purpose of the four steps of the QRM process: risk assessment, risk control, risk communication, and risk review.
- Explain the concept of Critical Control Points (CCPs) and their role in CCS implementation.
- Discuss the importance of reviewing and updating the CCS to ensure continuous improvement in contamination control.
Keywords
- EU GMP Annex 1
- Contamination Control Strategy
- Critical Control Points
- Quality Risk Management
- Regulatory Compliance
- Risk Assessment
- Risk Control
- Risk Communication
- Risk Management
- Risk Review
- Sterile Product Manufacturing
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen