Contamination Control Strategy – QRM in Practice
Unlocks the ‘how’ of using Quality Risk Management (QRM) to successfully devise, implement, and maintain your facility’s Contamination Control Strategy (CCS). Using the example of contamination control in a lyophilization process, the user explores and applies QRM steps, including risk analysis, risk control, and corrective action planning.
Interested in the ‘why’ of Annex 1 CCS requirements? Please see our companion module Contamination Control Strategy – Annex 1 and QRM!
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
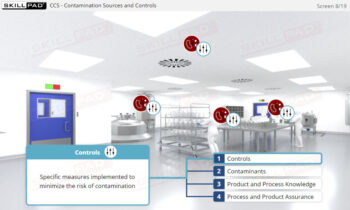
- Gain a comprehensive understanding of the EU GMP Annex 1 requirements for contamination control in sterile product manufacturing.
- Using a lyophilization process as a case study, acquire practical skills in using Quality Risk Management (QRM) principles to devise, implement, and maintain a Contamination Control Strategy (CCS).
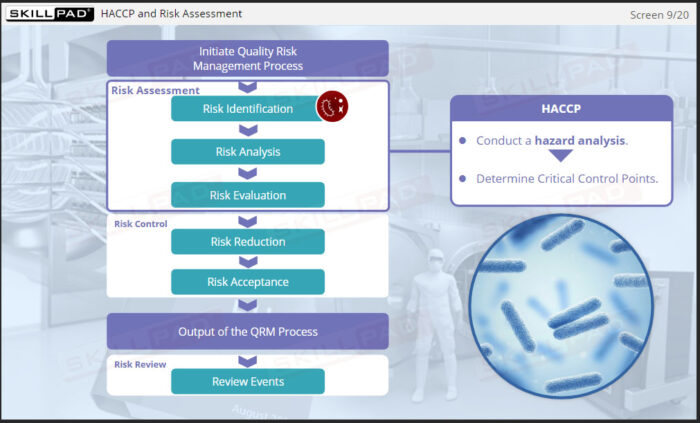
- Learn the tasks involved in applying HACCP to the Risk Assessment and Risk Control steps of QRM and recognize the importance of actively reviewing and updating a CCS as per Annex 1 requirements.
- Develop critical thinking skills to define Critical Control Points (CCPs) and provide examples of CCPs for a lyophilization process.
Learning Objectives
- Identify key Annex 1 requirements for contamination control in sterile product manufacturing.
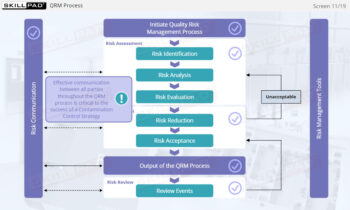
- Explain the application of Quality Risk Management (QRM) principles to devising, implementing, and maintaining a CCS for lyophilization.
- Describe the tasks involved in applying HACCP to the Risk Assessment step of QRM.
- Define Critical Control Point (CCP) and provide examples of CCPs for a lyophilization process.
- Describe tasks for applying HACCP to the Risk Control step of QRM.
- Recognize the importance of actively reviewing and updating a CCS as per Annex 1 requirements.
Keywords
- Contamination Control Strategy
- Critical Control Point
- EU GMP Annex 1
- HACCP
- Lyophilization
- Quality Risk Management
- Risk Assessment
- Risk Control
- Regulatory Compliance
- Risk Management
- Sterile Product Manufacturing
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen