Crystallization
An introduction to the principles and techniques of crystallization used in API manufacturing. This module covers the formation of crystals, the role of solvents, and key process variables, as well as common issues that may arise during crystallization and the steps taken to resolve them, including the use of seed crystals and recrystallization techniques.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
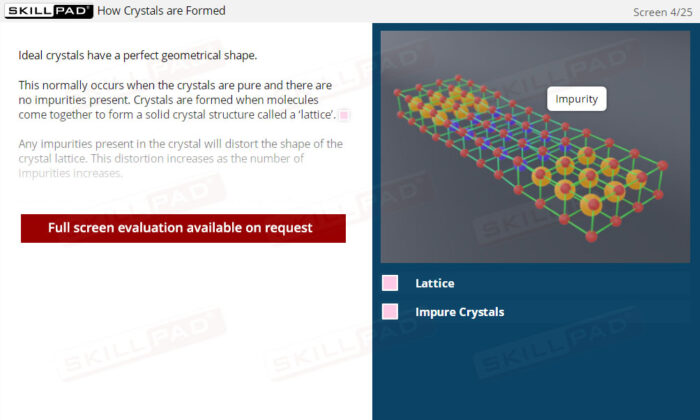
- Understand the Basics of Crystallization: Learn what crystals are, how they form, and their importance in the pharmaceutical manufacturing process.
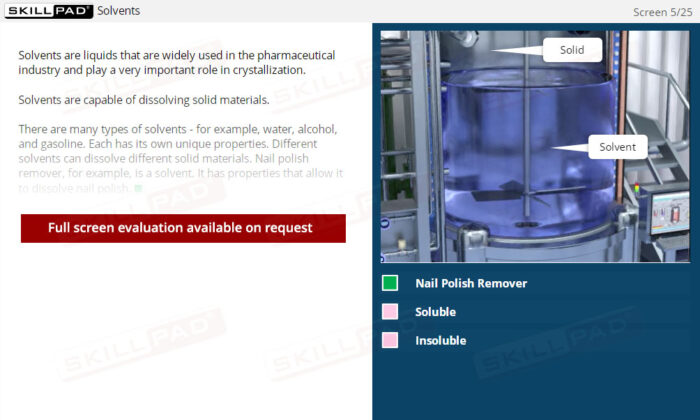
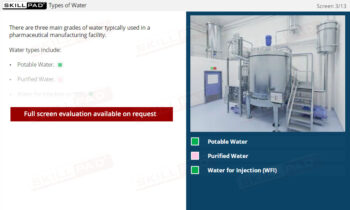
- Explore Solvent Selection and Usage: Discover how solvents are chosen based on their ability to dissolve specific substances and how changes in solvent conditions can affect crystallization outcomes.
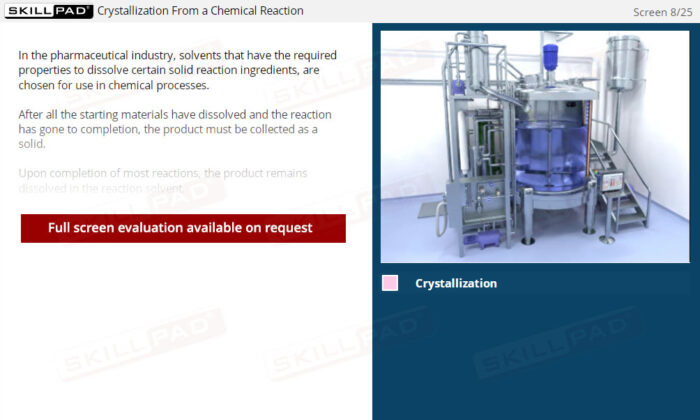
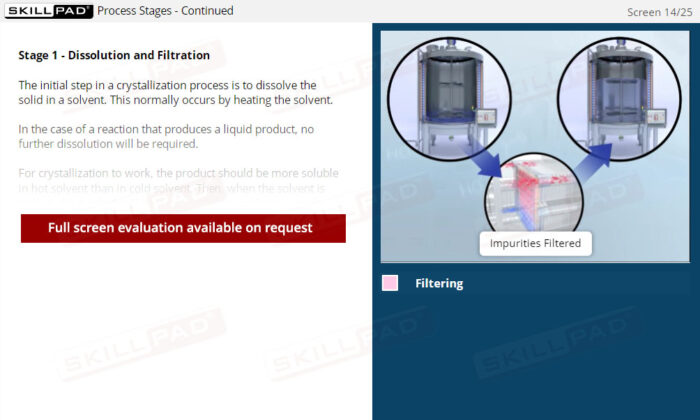
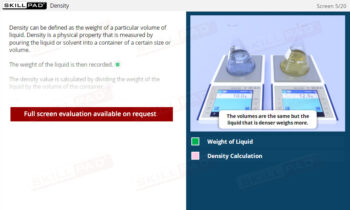
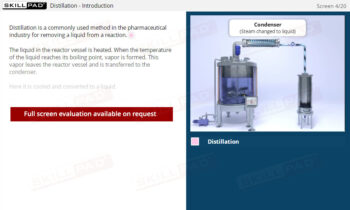
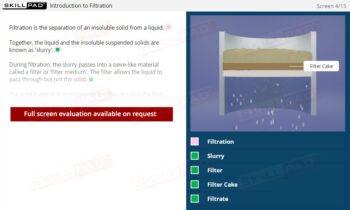
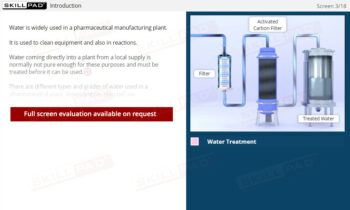
- Identify the Stages of Crystallization: Understand the key steps in the crystallization process, including dissolution, filtration, cooling, and the collection and washing of crystals, to support the production of high-quality products.
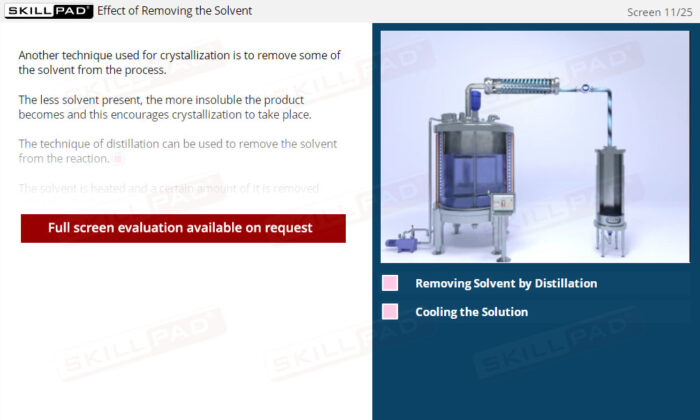
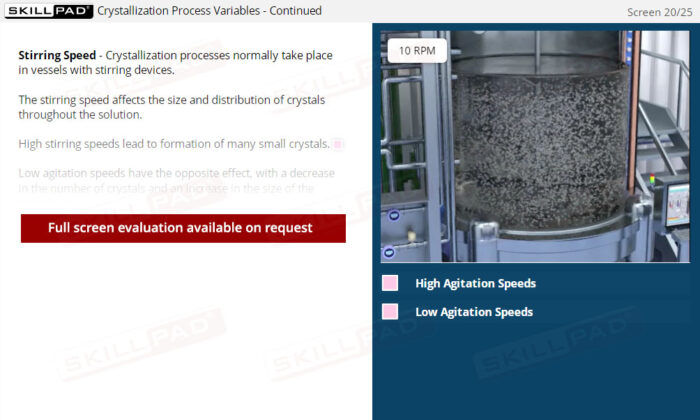
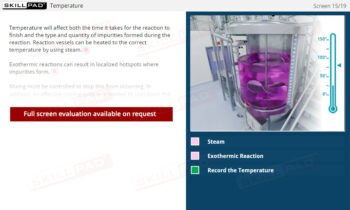
- Manage Key Process Variables: Learn how factors such as temperature, cooling rate, stirring speed, and solvent volume impact crystallization, and how to adjust these variables to help optimize yield and purity.
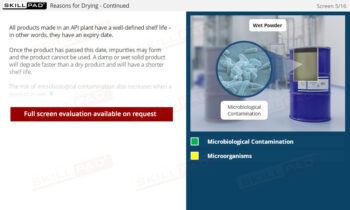
- Address Crystallization Challenges: Gain practical knowledge on how to handle common crystallization issues, such as impurity entrapment, by using techniques like seed crystals and recrystallization to improve product purity.