Data Integrity for GxP Regulated Industries
A comprehensive examination of the importance of data integrity in GxP-regulated industries, focusing on the critical role data plays in ensuring product quality and safety. The module explores key regulations such as 21 CFR Part 11, outlines the consequences of data integrity failures, and provides practical measures for maintaining secure and accurate records. Using real-world scenarios, learners will gain a deep understanding of the principles and best practices necessary to safeguard data throughout its life cycle and in compliance with regulatory requirements.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Strategic
Description
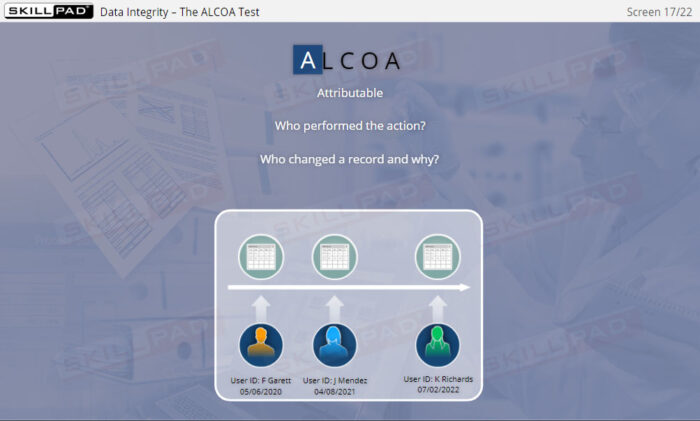
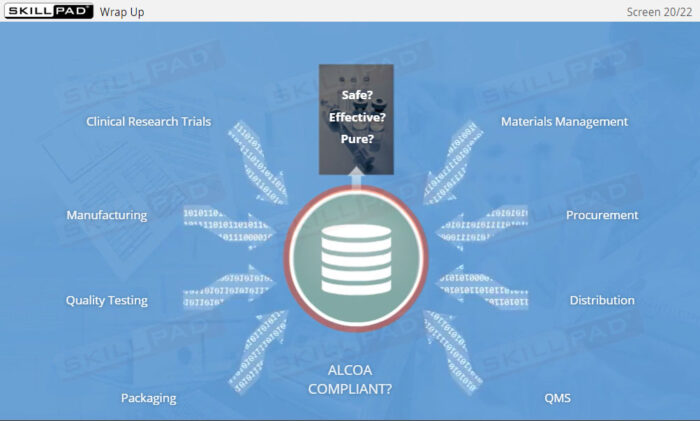
- Understand Data Integrity Principles and ALCOA+: Gain a deep understanding of data integrity and the ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) to ensure compliance and reliability in GxP-regulated environments.
- Navigate Key Regulations: Explore critical regulations such as 21 CFR Part 11 and Annex 11, and how they enforce electronic recordkeeping and signature integrity, aligning with ALCOA+ standards.
- Implement ALCOA-Based Data Integrity Practices: Learn strategies for preventing and addressing data integrity issues, including the use of audit trails, data life cycle management, and adherence to ALCOA+ principles.
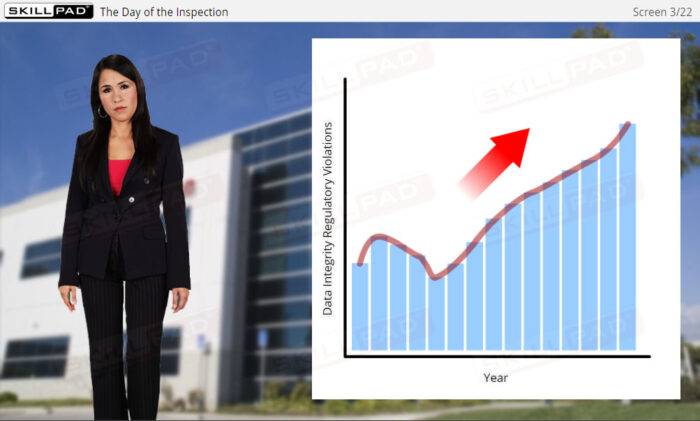
- Recognize the Consequences of Non-Compliance: Recognize the legal, financial, and reputational risks associated with data integrity failures at both individual and organizational levels.
Learning Objectives
- Explain what is meant by ‘data integrity’ in relation to GxP records.
- Explain why data integrity is important in assuring product quality.
- Describe some of the consequences of data integrity failings.
- State where data integrity regulations apply in regulated industries.
- Describe the role of 21 CFR 11 in data integrity compliance.
- Explain the term ‘data life cycle’.
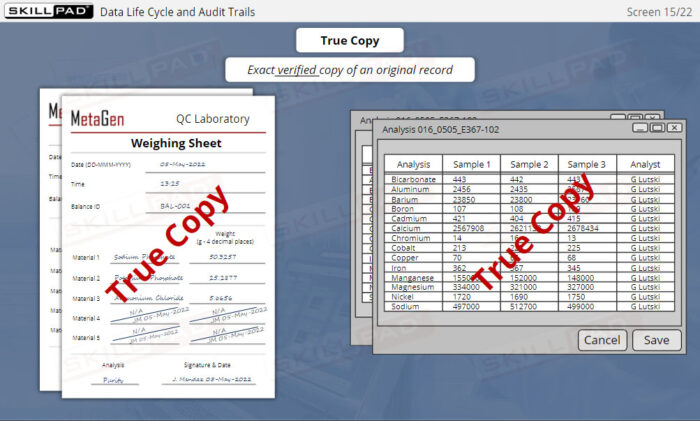
- Explain what is meant by an ‘original record’.
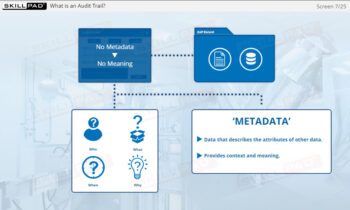
- Explain the purpose of an audit trail.
- Explain each of the ALCOA Data Integrity requirements.
- Describe some practical measures for ensuring data integrity in GxP regulated industries.
Keywords
- 21 CFR Part 11
- ALCOA Principles
- Audit Trail
- Biopharmaceuticals
- Continuous Improvement
- Data Integrity
- Data Life Cycle
- Electronic Records
- Good Manufacturing Practice (GMP)
- GxP Compliance
- Non-Compliance
- Quality Assurance
- Quality Management Systems (QMS) Regulatory Standards
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen