Data Integrity – GxP Audit Trail Requirements
An exploration of the essential components of audit trails within GxP-regulated environments, this module covers the definition, purpose, and regulatory requirements of audit trails, emphasizing their role in ensuring data integrity. Participants will learn to distinguish between data and system audit trails, recognize essential characteristics of audit trail entries, and understand the implications of audit trail violations. The module also highlights the importance of regular and scheduled audit trail reviews and introduces concepts such as Data Integrity Risk Assessment, data criticality, and data risk.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Strategic
Description
- Recognize the Importance of Audit Trails: Gain insights into the critical role of audit trails in maintaining data integrity and ensuring compliance within GxP-regulated industries.
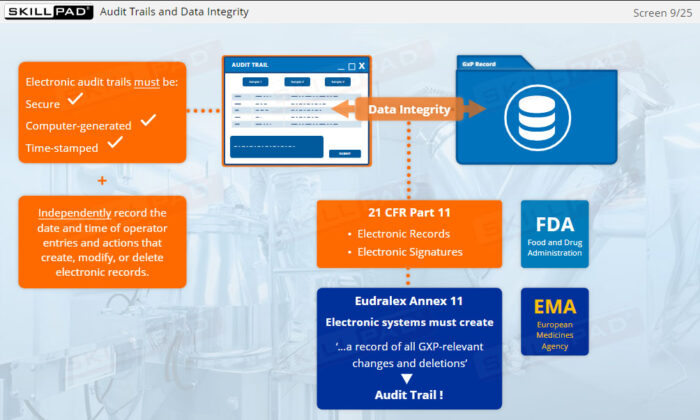
- Navigate Regulatory Standards for Audit Trails: Explore key regulatory requirements from global agencies such as the FDA and EMA to ensure compliance with industry standards.
- Identify and Address Audit Trail Violations: Learn to recognize common regulatory violations and apply a proactive approach to compliance and risk management in data handling.
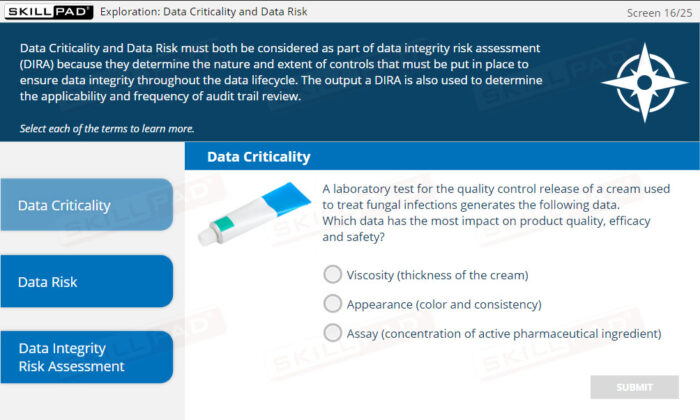
- Assess Data Integrity Risks Effectively: Understand the principles of Data Integrity Risk Assessment to evaluate data criticality, assess risks, and make informed decisions regarding audit trail reviews.
Learning Objectives
- Define ‘audit trail’.
- Explain the purpose of an audit trail with respect to ALCOA+ data integrity principles.
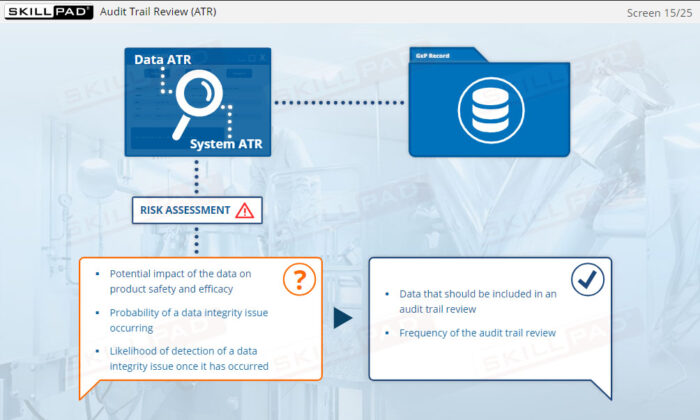
- Describe the two different types of audit trail.
- List the essential characteristics of a complete audit trail entry.
- Give examples of typical audit trail regulatory deficiencies.
- Explain the purpose of an audit trail review.
- Explain what is meant by Data Integrity Risk Assessment.
- Describe the general regulatory requirements for audit trails.
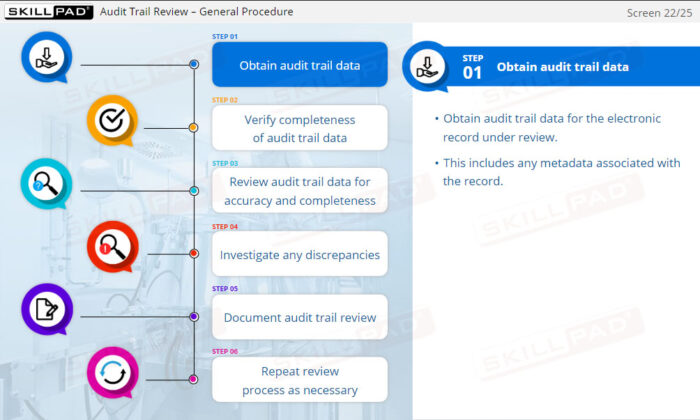
- Explain what is involved in ‘regular’ and ‘scheduled’ audit trail reviews.
- Describe the key components of an audit trail review.
- Describe specific checks that might be done as part of ATR.
Keywords
- ALCOA+
- Audit Trail Review
- Audit Trail Violations
- Compliance
- Data Integrity
- Electronic Records
- Eudralex Annex 11
- FDA 21 CFR Part 11
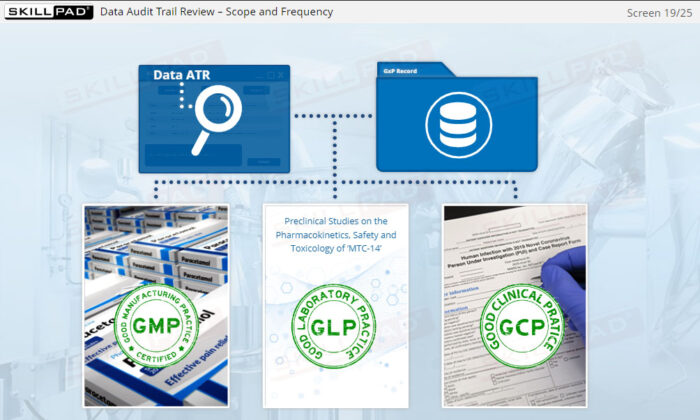
- GxP (Good Laboratory, Clinical, and Manufacturing Practices)
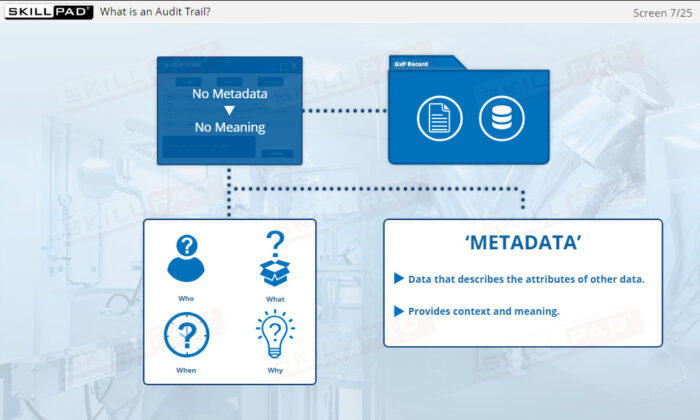
- Metadata
- Regulatory Requirements
- Risk Assessment
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen