Dissolution Testing
A comprehensive guide to the practical aspects of dissolution testing, covering the essential steps from setting operational parameters and adding dosage forms to sampling, data handling, and result verification. The module details procedures for both manual and automatic sampling, emphasizes critical timing elements, and explains the calculations necessary to determine the concentration and percent label claim of active ingredients. It also clarifies the criteria for when retesting may be necessary, ensuring compliance with standard operating procedures and pharmacopeial standards.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
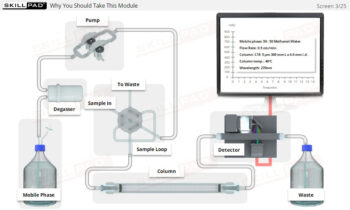
- Mastery of Dissolution Apparatus Setup: Develop a thorough understanding of how to set key parameters such as rotation speed, temperature, and dissolution time, ensuring consistent and accurate test execution.
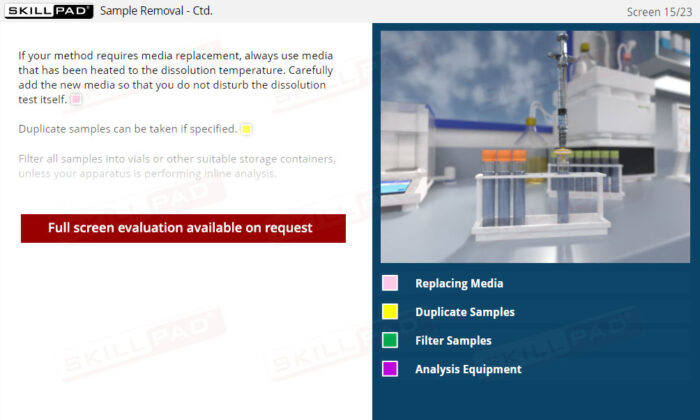
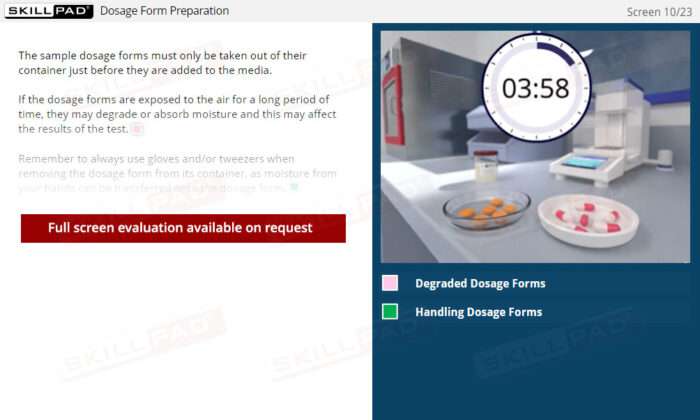
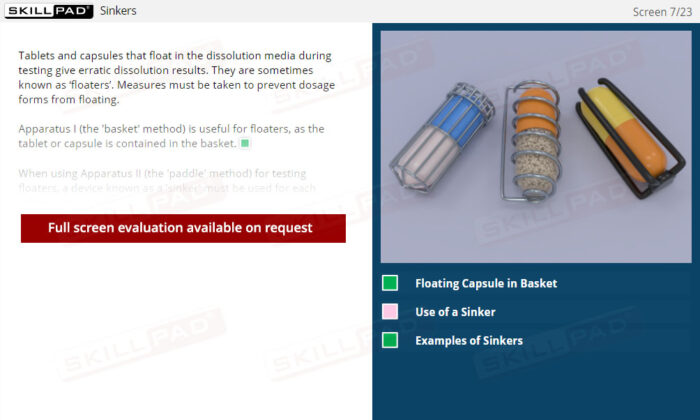
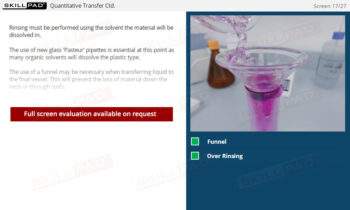
- Confidence in Dosage Form Handling: Learn best practices for dosage form preparation and addition, including techniques to prevent floating and methods for staggered or simultaneous addition.
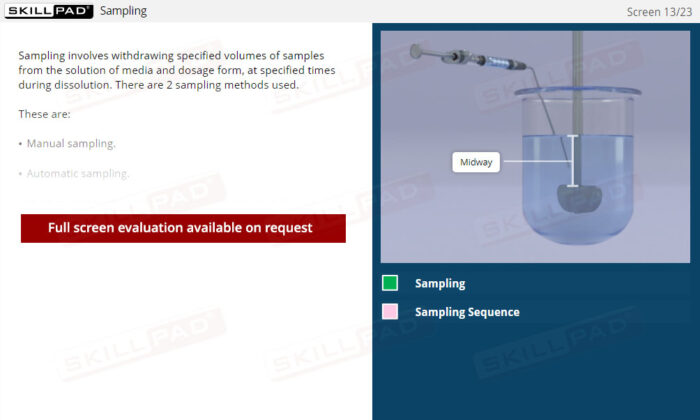
- Efficiency in Sampling Methods: Gain proficiency in both manual and automatic sampling, focusing on precise timing and sample volume management to maintain test validity.
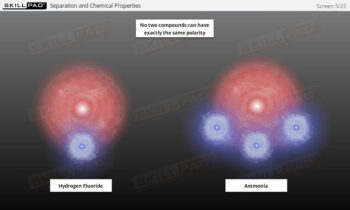
- Accuracy in Critical Calculations: Strengthen your skills in calculating concentration, weight of active dissolved, and percent label claim, crucial for validating dissolution results.
- Compliance with Acceptance Criteria: Understand how to compare results against pharmacopeial standards, interpret USP acceptance stages, and determine when to conduct retesting.
- Practical Problem-Solving Skills: Equip users with the ability to identify and address potential issues, such as missing samples or improper storage, to ensure the reliability of test outcomes.
Learning Objectives
- List the operating parameters that are set on a dissolution apparatus.
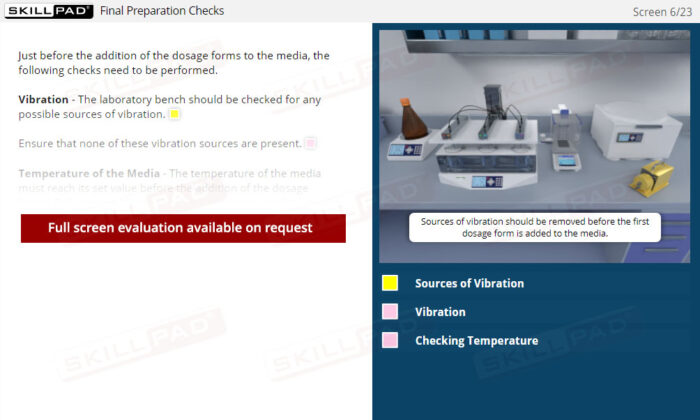
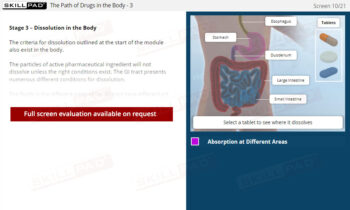
- Describe the final preparation checks done before adding dosage forms to dissolution media.
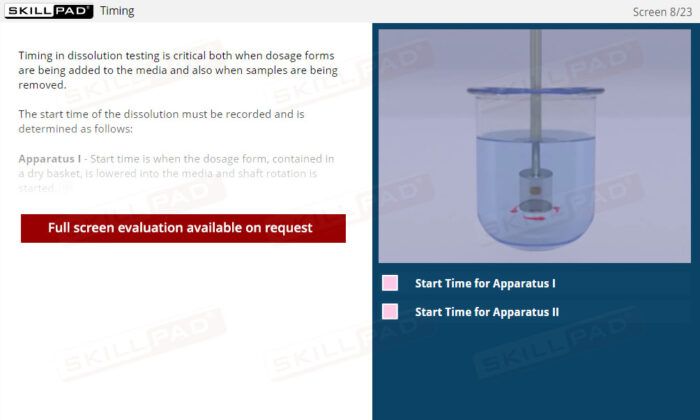
- Describe how dissolution start time is handled for a Type 1 and Type 2 dissolution apparatus.
- Explain the difference between staggering and simultaneous dosage form addition.
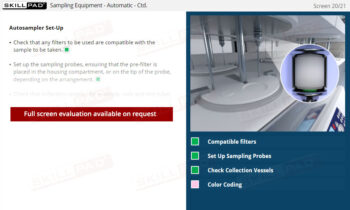
- Describe the procedures involved in manual and automatic sampling.
- Perform calculations for concentration of active in the vessel, weight of active dissolved, and percent label claim.
- Compare results against USP acceptance criteria.
- List examples of when retesting can be performed.
Keywords
- Active Concentration Calculation
- Automatic Sampling
- Dissolution Apparatus
- Dissolution Testing
- Dosage Form Addition
- Manual Sampling
- Percent Label Claim
- Pharmacopeial
- Regulatory Compliance
- Retesting Criteria
- Sampling Procedures
- USP Acceptance Criteria
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen