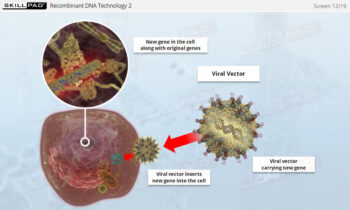
Downstream Processing: Centrifugation
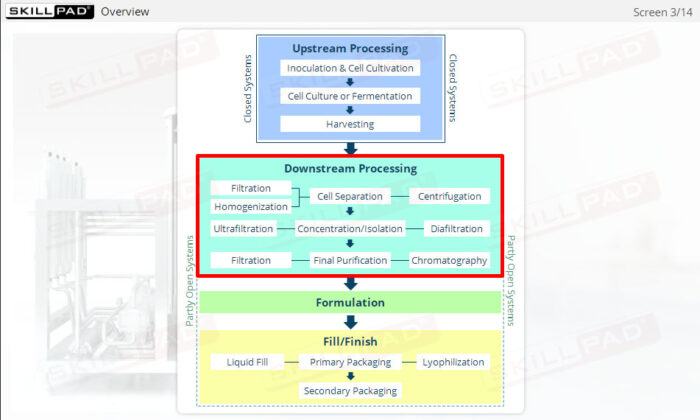
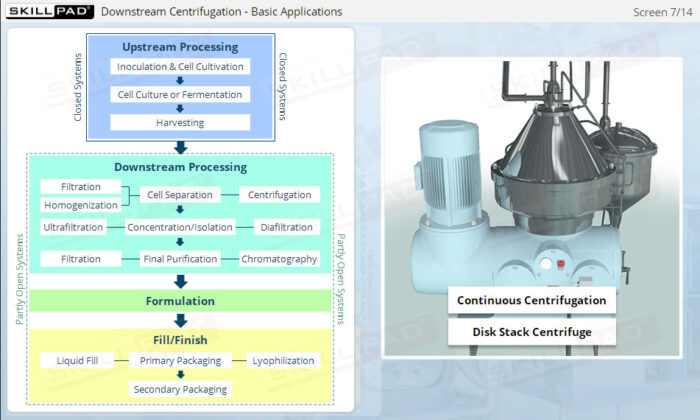
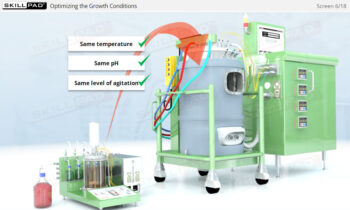
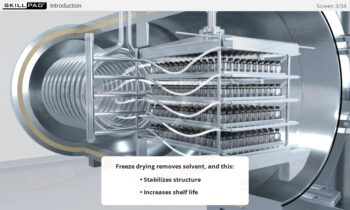
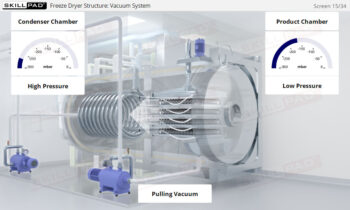
Describes what centrifugation is and the stages of biopharmaceutical downstream processing where it can be used. Primary cell separation using a Disk Stack Centrifuge, and final purification using Ultracentrifugation are explained both in terms of equipment and process.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
- Gain a comprehensive understanding of the purpose and principles of centrifugation in downstream processing.
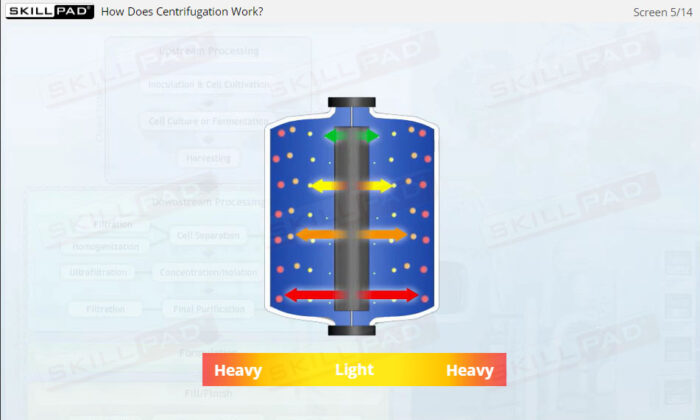
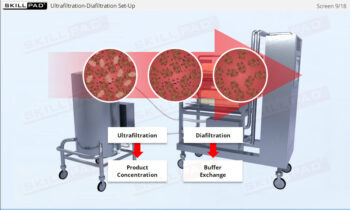
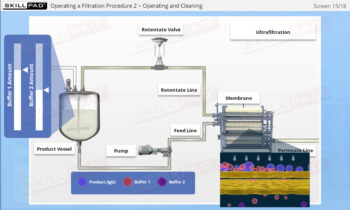
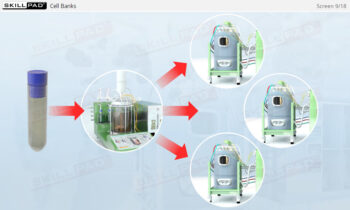
- Understand how centrifugation is used to separate components based on size and density, allowing for faster and more efficient processing of large volumes of harvest fluids.
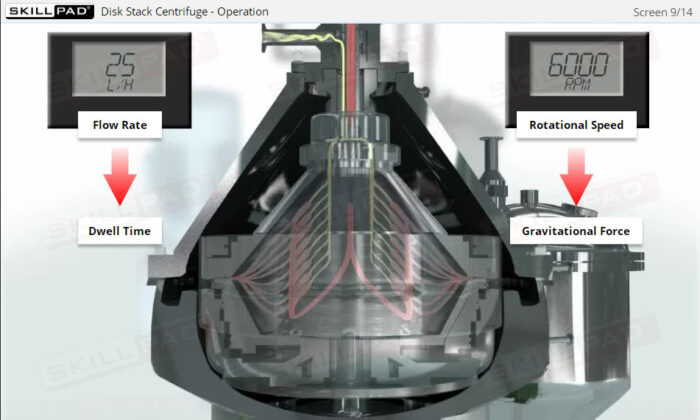
- Learn the structure and operational aspects of disk stack centrifuges, including flow rate control and ejection processes, to ensure continuous and effective separation in manufacturing environments.
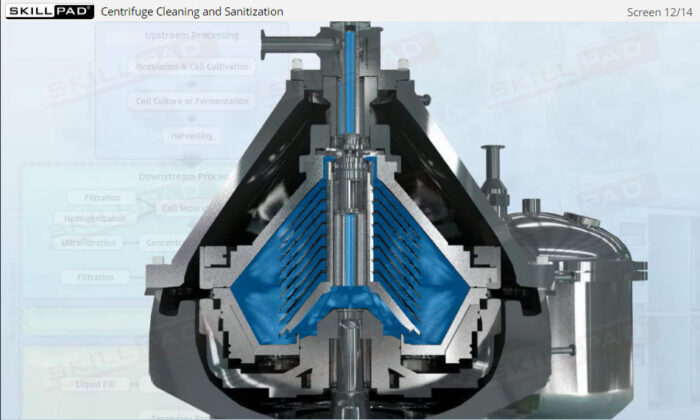
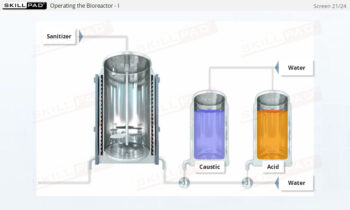
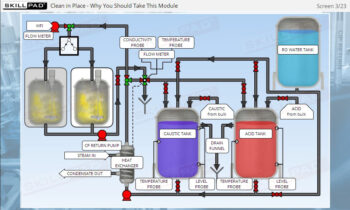
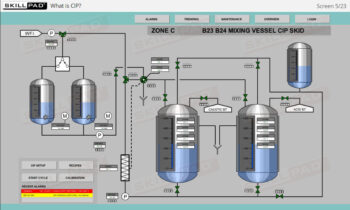
- Learn the critical importance of cleaning and sanitization, ensuring compliance with biopharmaceutical industry standards to prevent microbial contamination and maintain product quality.
Learning Objectives
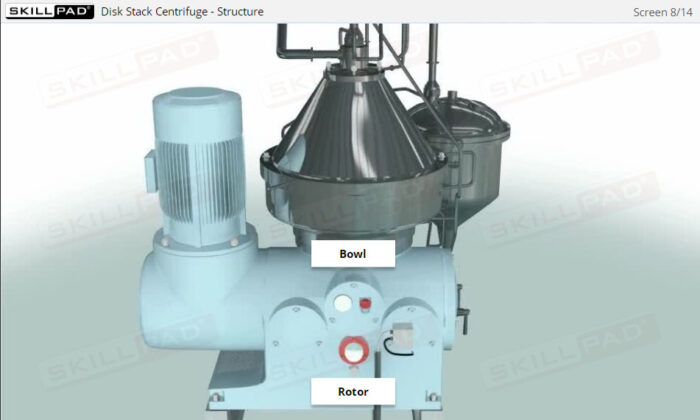
- Describe the structure, operating principles and applications of a disk stack centrifuge.
- Describe the structure, operating principles and applications of an ultracentrifuge.
- Explain the principle of centrifugation.
- Explain why cleaning and sanitization of centrifuges is so critical.
- Explain why purification is critical in biopharmaceutical manufacturing.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen