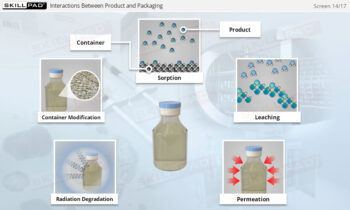
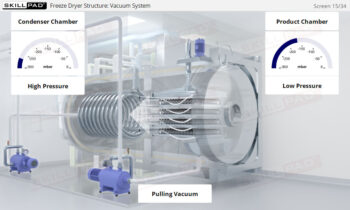
Downstream Processing: Ultrafiltration and Diafiltration
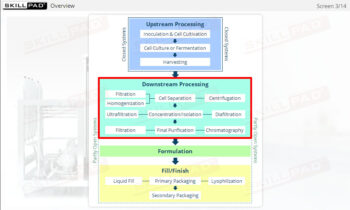
Describes the downstream manufacturing processes of ultrafiltration and diafiltration with an emphasis on post-harvest volume reduction and concentration for therapeutic protein products. The components of an UF/DF skid and control of the UF/DF process are also described.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
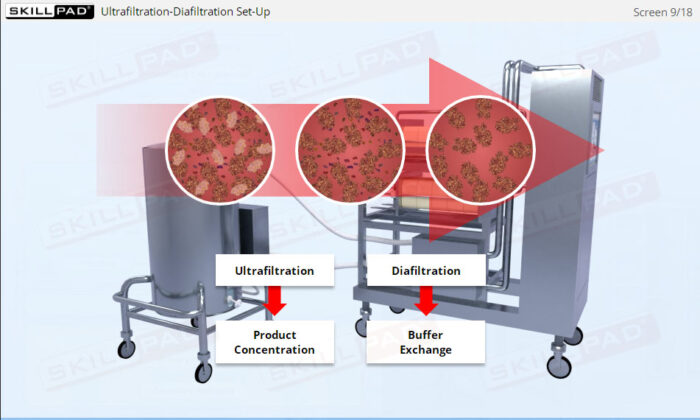
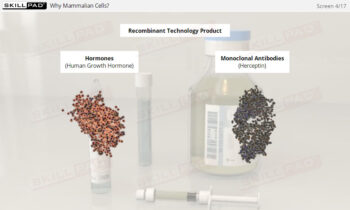
- Gain in-depth knowledge of the role of ultrafiltration and diafiltration in protein purification, including the differences and advantages of normal flow and tangential flow filtration.
- Enhance your technical vocabulary and understanding of key terminology such as molecular weight cut-off, transmembrane pressure, retentate, and permeate.
- Explore the components of UF/DF skids and their functions.
- Identify critical process parameters and understand the role of in-process checks, quality tests, and troubleshooting during UF/DF operations.
- Gain practical knowledge of the set-up, operation, and cleaning of ultrafiltration and diafiltration systems.
- Understand the safety requirements associated with UF/DF operations and learn how to maintain high standards of cleanliness and integrity in biopharmaceutical manufacturing environments.
Learning Objectives
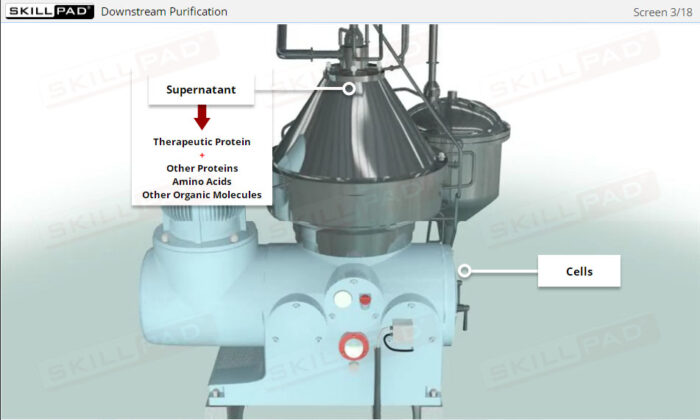
- Explain the role of filtration in protein purification methods.
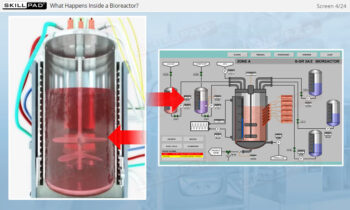
- Describe the role of ultrafiltration / diafiltration (UF/DF) in downstream processing.
- Describe the difference between Normal Flow Filtration (NFF) and Tangential Flow Filtration (TFF).
- List the advantages of TFF over NFF.
- Explain the terms molecular weight cut off and transmembrane pressure.
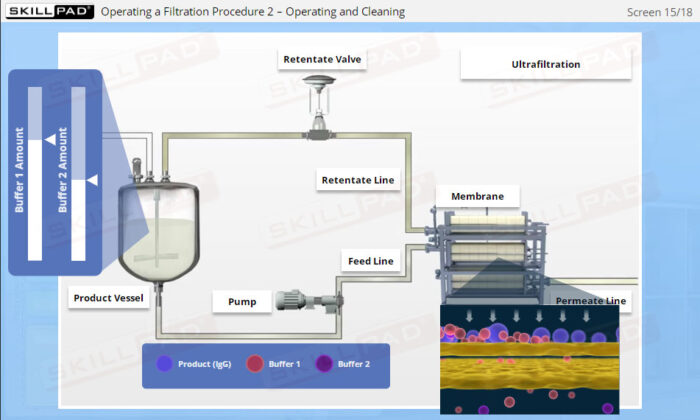
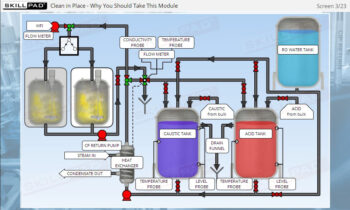
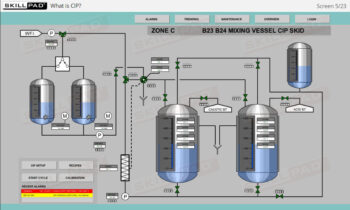
- Identify important components of a UF/DF skid and their functions.
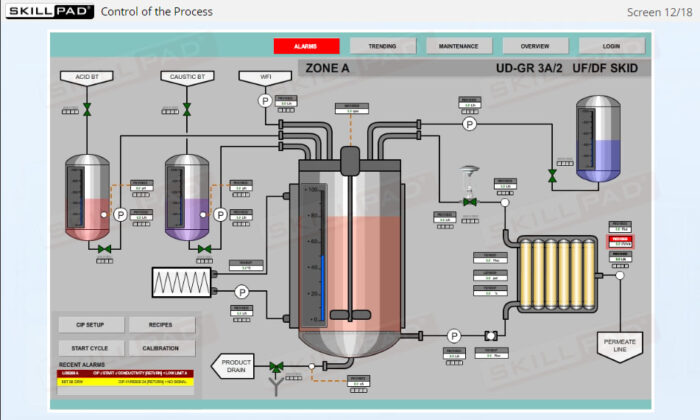
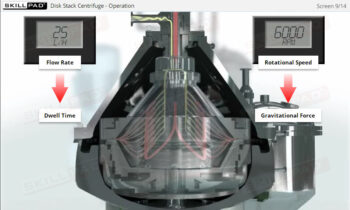
- List and explain the critical process parameters involved in a UF/DF operation.
- Describe the in-process checks and quality tests that are carried out during a UF/DF operation.
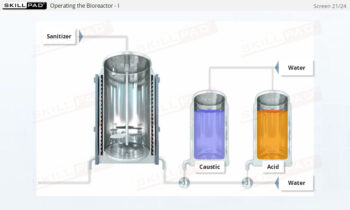
- Describe how a UF/DF skid is cleaned.
- Describe the safety requirements associated with UF/DF.
- List some common problems encountered when operating UF/DF.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen