Dress Codes for Finished Dose Manufacturing
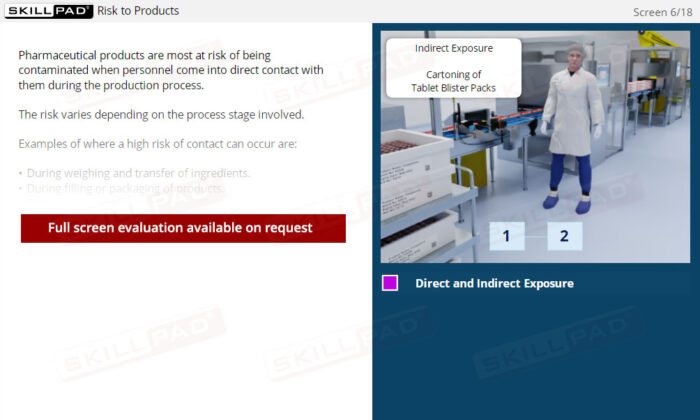
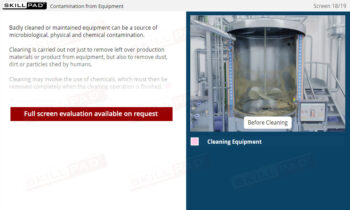
An overview of contamination prevention in finished dose pharmaceutical manufacturing, highlighting the types and sources of contamination—microbiological, physical, and chemical. This module explains critical practices to minimize contamination risks, including hygiene, cleanliness, production protocols, and the use of Personal Protective Equipment (PPE). Emphasis is placed on understanding the role of facility personnel and environmental controls in safeguarding product quality and patient safety.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
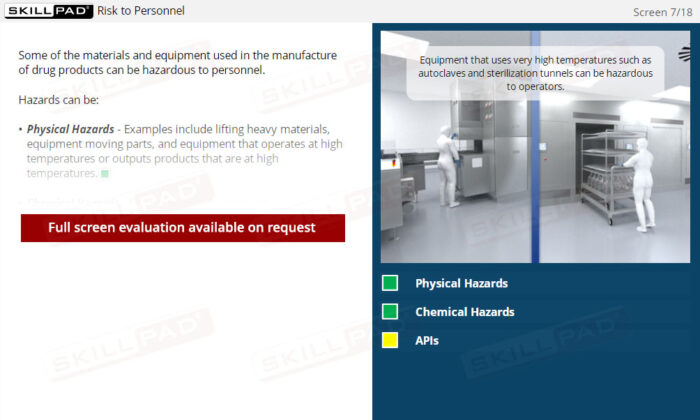
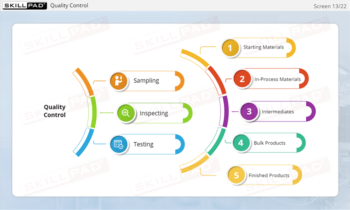
- Identify Contamination Sources: Gain foundational knowledge of different types of contaminants and primary contamination sources within a finished dose manufacturing facility.
- Learn Contamination Prevention Techniques: Understand practical steps to minimize contamination risks, such as personal hygiene, equipment cleaning, and maintenance practices.
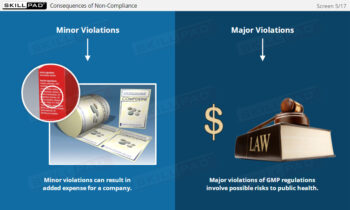
- Understand the Proper Use of PPE: Learn the importance and correct usage of PPE to protect both products and personnel and ensure compliance with GMP.
- Build Awareness of GMP Hygiene Standards: Develop essential hygiene habits and practices to prevent contamination in pharmaceutical production environments.
Learning Objectives
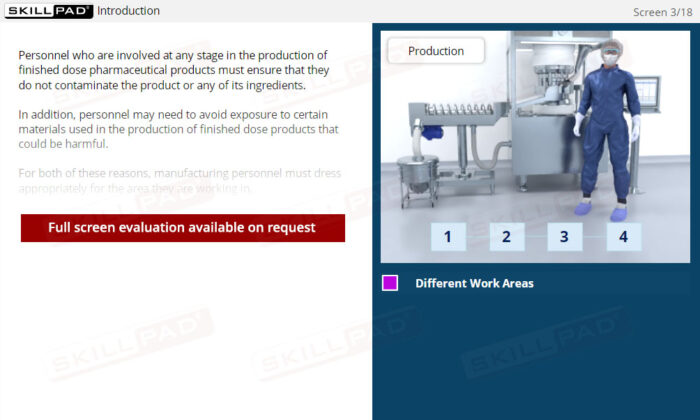
- Explain why dress codes exist in pharmaceutical manufacturing facilities.
- Name the different types of dress codes in a finished dose manufacturing plant.
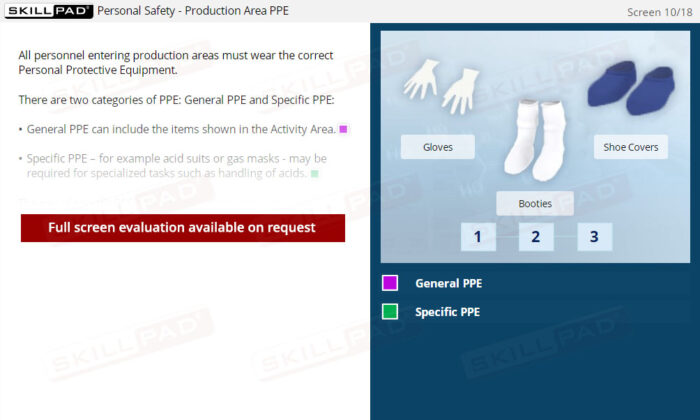
- Recognize the different types of Personal Protective Equipment (PPE).
- Describe why following dress codes is essential in a finished dosage form plant.
Keywords
- Air Filtration Systems
- Chemical Contaminants
- Contamination Prevention
- Dress Code
- Environmental Controls
- Finished Dose Manufacturing
- Good Manufacturing Practices (GMP)
- HVAC Systems
- Hygiene Practices
- Microbiological Contaminants
- Personal Protective Equipment (PPE)
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen