Errors in GMP Regulated Manufacturing
A comprehensive look at errors in GMP-regulated environments, examining the types of errors that occur, their root causes, and the critical distinction between errors and violations. This module covers error management strategies that include both reactive and proactive approaches, helping to ensure regulatory compliance and product quality.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Strategic
Description
- Understand the Complex Nature of Errors: Learn to distinguish between skill-based, rule-based, and knowledge-based errors, and gain insights into the regulations that govern error investigation in GMP environments.
- Identify and Address Root Causes Effectively: Explore why ‘human error’ is not an acceptable root cause, and discover various tools and methodologies, such as Root Cause Analysis (RCA), to identify underlying issues in work systems.
- Develop Skills in Error Management: Learn how to identify, classify, and manage errors effectively, helping to prevent future incidents and ensure continuous improvement in GMP compliance.
- Enhance Compliance with Industry Standards: Gain practical knowledge of the regulatory requirements surrounding errors and violations, contributing to robust quality systems within your organization.
- Apply Learning Through Scenario-Based Exercises: Reinforce understanding of error classification and management with realistic scenarios, facilitating competent handling and mitigation of errors in the workplace.
Learning Objectives
- Define ‘error’ as it applies to human behavior in GMP-regulated environments.
- Explain the relevant industry regulations that relate to errors and their investigation.
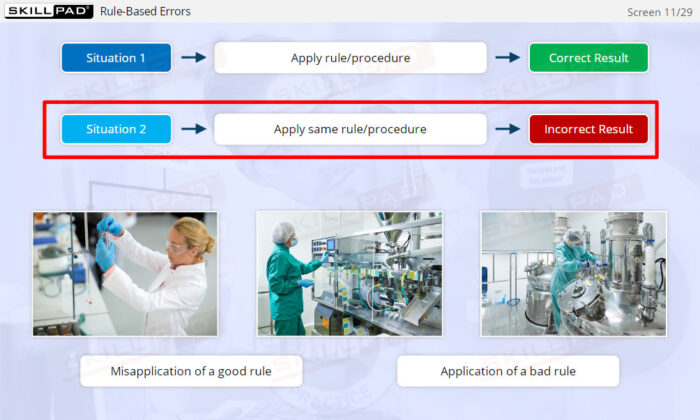
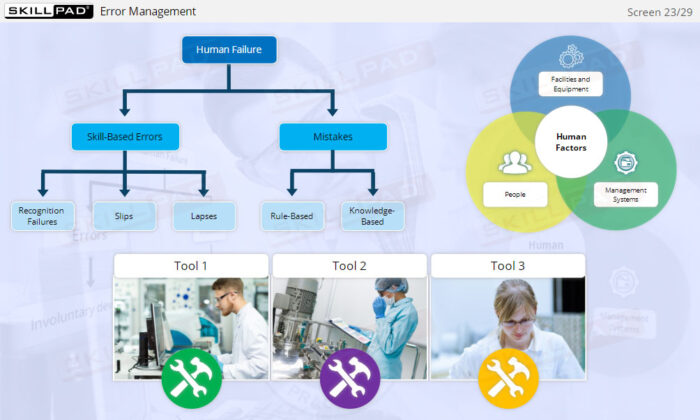
- Distinguish between skill-based, rule-based, and knowledge-based errors.
- Provide examples of errors that can arise in a GMP manufacturing environment.
- Explain why ‘human error’ is not a valid root cause.
- Outline various approaches for determining root cause(s) of errors.
- Explain reactive and proactive approaches to error management.
Keywords
- CAPA System
- Error Classification
- Error Management
- FDA Regulations
- GMP Manufacturing
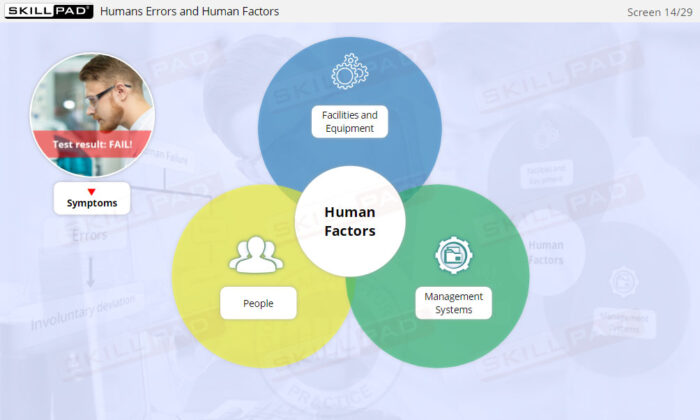
- Human Factors
- Knowledge-Based Errors
- Proactive Approaches
- Quality Control, Reactive Approaches
- Regulatory
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen