Executive Responsibility in Pharma Manufacturing
This module provides executive management in pharmaceutical manufacturing with a comprehensive understanding of their legal and regulatory responsibilities under the U.S. Food, Drug, and Cosmetic Act (FD&C Act). It explores the role of the FDA in ensuring compliance, the different types of inspections conducted, and the serious consequences of non-compliance— including FDA 483s, Warning Letters, and potential legal action. The module also emphasizes the central role of a properly functioning Quality System in maintaining compliance and preventing regulatory violations.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand Executive Accountability in GMP Compliance: Gain insight into how the FD&C Act holds executive management legally responsible for compliance with cGMP regulations, ensuring you fully grasp your obligations in a pharmaceutical manufacturing setting.
- Recognize the Role of the FDA in Regulatory Enforcement: Learn about the structure and function of the FDA, the types of inspections conducted, and how regulatory actions such as FDA 483s, Warning Letters, and Consent Decrees can impact your organization.
- Mitigate the Risks of Non-Compliance: Understand the serious consequences of failing to comply with FDA regulations— including product seizures, loss of market approval, and even criminal prosecution—and how to prevent these risks through proactive compliance.
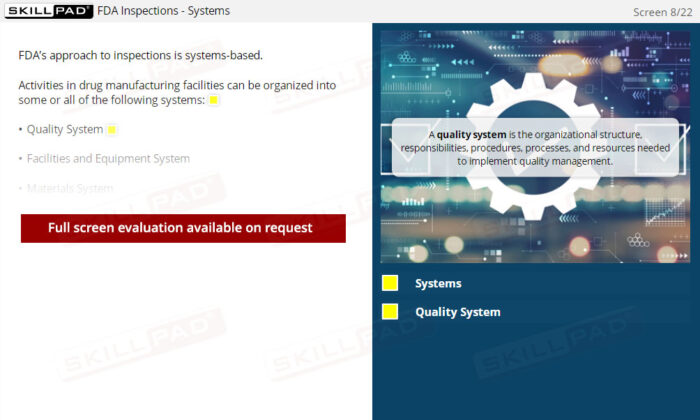
- Ensure a Well-Functioning Quality System: Discover the critical components of an effective Quality System, how it serves as the foundation for GMP compliance, and how executive leadership plays a pivotal role in ensuring its effectiveness.
- Prepare for FDA Inspections: Learn how FDA inspectors assess Quality Systems, review documentation, and identify deficiencies—so you can anticipate expectations and maintain regulatory readiness.
- Strengthen Documentation Practices for Compliance: Understand the vital role of documentation in demonstrating compliance, and ensure your organization maintains accurate and complete records to satisfy FDA expectations.
- Enhance Compliance Strategies: Explore best practices for implementing corrective and preventive actions (CAPAs), conducting internal audits, and ensuring ongoing Quality System effectiveness to avoid regulatory issues before they arise.
Learning Objectives
- Explain the relationship between a pharma company’s Executive Management and the FDA.
- Describe the purpose and function of the FDA.
- Describe 3 types of FDA inspection and the purpose of each.
- Explain FDA 483s, Warning Letters, and Consent Decrees.
- Explain the terms ‘Quality Policy’ and ‘Quality System’.
- Describe the characteristics of a properly functioning Quality System.
- Explain why documentation is so important in a Quality System.
Keywords
- cGMP Regulations
- Consent Decrees
- Corporate Accountability
- Documentation and Compliance
- Executive Responsibility
- FDA Compliance
- FDA Inspections
- FDA 483s and Warning Letters
- Pharmaceutical Manufacturing
- Quality Assurance and Control
- Quality System Management
- Regulatory Risk Management
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen