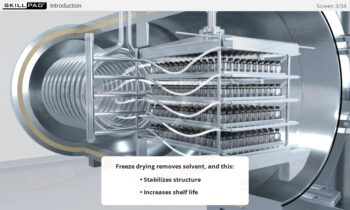
Fermentation in Biopharmaceutical Manufacturing
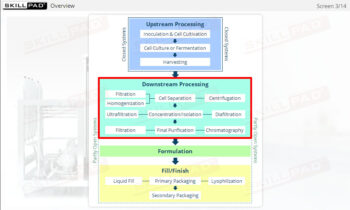
Describes how microorganisms are used in fermentation processes as part of biopharmaceutical manufacturing. Areas covered include growth phases and characteristics and conditions, cell banks, media, bioreactors and modes of operation, and the importance of sterility.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
- Gain a solid foundation in fermentation, including microbial growth stages and the use of bioreactors, to produce high-value biopharmaceuticals.
- Learn to differentiate between batch, continuous, and fed-batch fermentation modes and apply best practices in media selection and inoculation techniques.
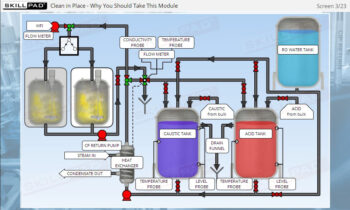
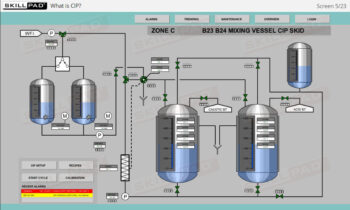
- Recognize the critical role of equipment cleaning and sterilization to ensure successful and contamination-free fermentation processes.
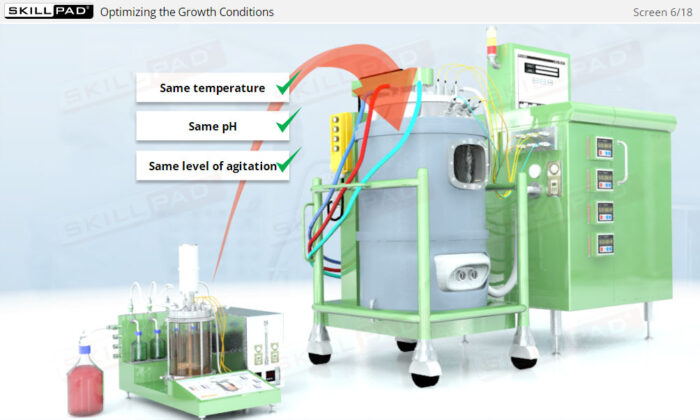
- Explore the role of microbial growth kinetics and process parameters in optimizing fermentation conditions for maximum yield and efficiency.
- Equip yourself with knowledge to comply with industry standards and regulations, ensuring high-quality and consistent biopharmaceutical production.
Learning Objectives
- Explain what is meant by ‘fermentation’.
- List the different stages of microbial growth.
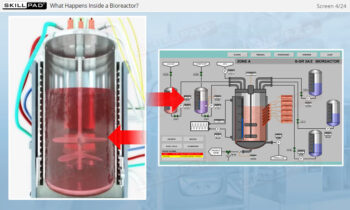
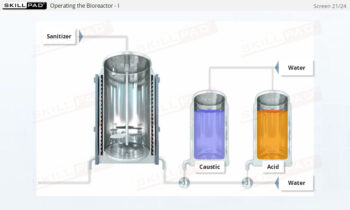
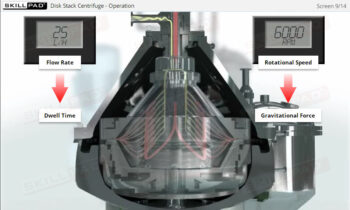
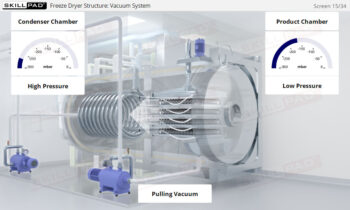
- Describe the structure of a typical bioreactor used for fermentation.
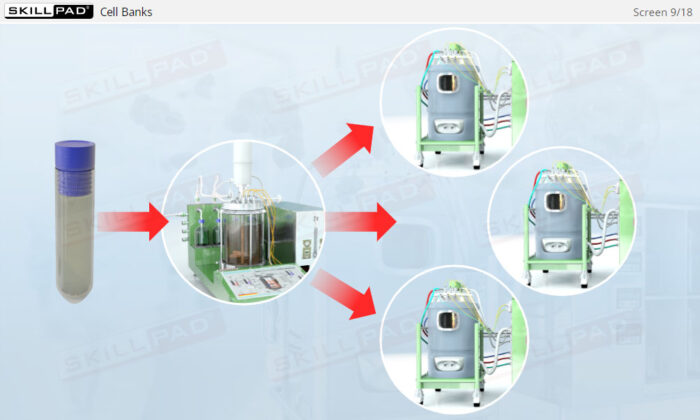
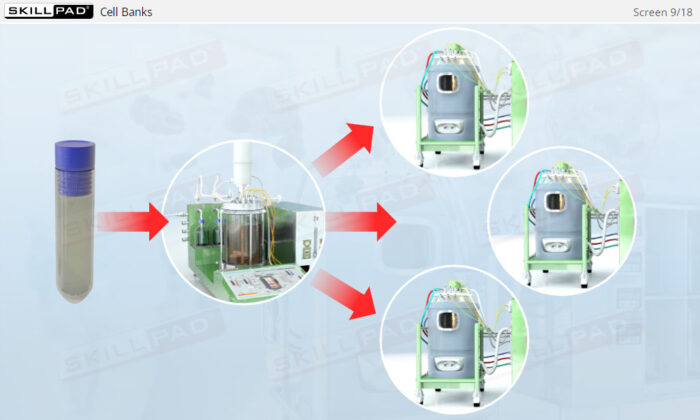
- Explain the concept of a cell bank and how an inoculation procedure is performed.
- Distinguish between defined and undefined media used in a fermentation process.
- Distinguish between batch and continuous modes of fermentation.
- Explain why cleaning and sterilization of equipment are so critical in fermentation.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen