Formulation & Packaging in the Biopharmaceutical Industry
Provides an overview of the principles and practices of formulation and packaging processes in a modern biopharmaceutical manufacturing facility.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
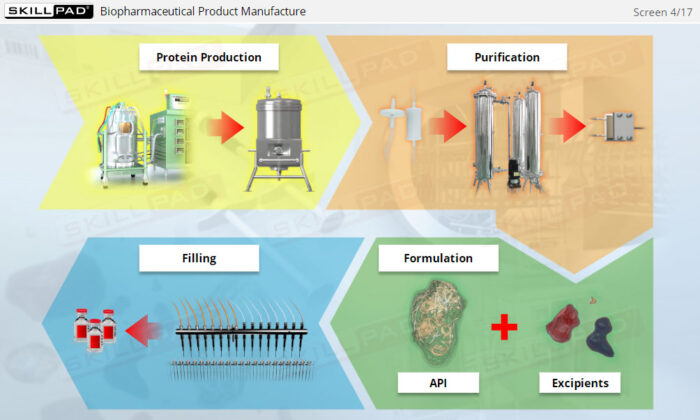
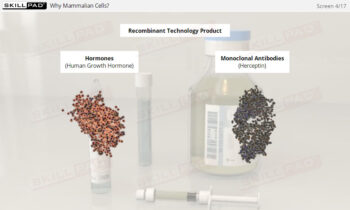
- Gain a thorough understanding of the formulation process, including the roles of APIs and excipients in creating safe and effective biopharmaceutical products.
- Identify key formulation considerations such as stability and purity and understand how different excipients function to maintain product integrity.
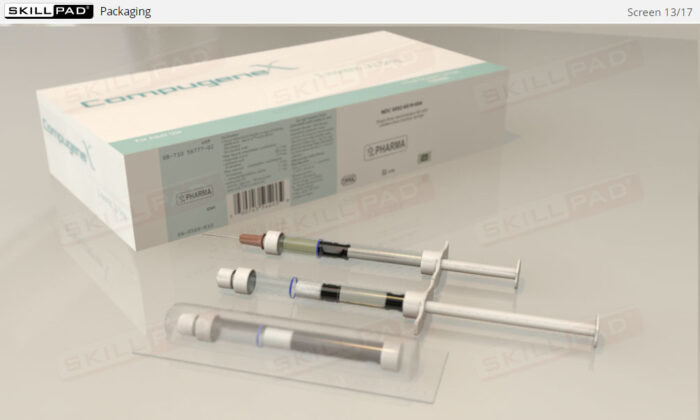
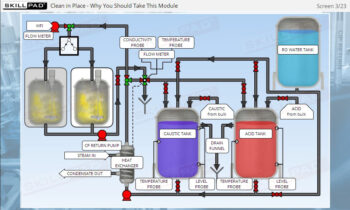
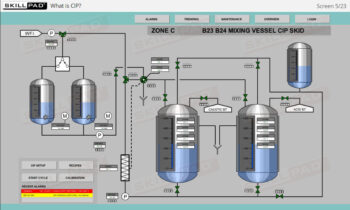
- Learn about the critical factors in selecting appropriate packaging types to prevent contamination, damage, and degradation of biopharmaceutical products during transport and storage.
- Understand how formulation and packaging contribute to maintaining the safety and efficacy of biopharmaceutical products.
Learning Objectives
- Describe the purpose of biopharmaceutical product formulation.
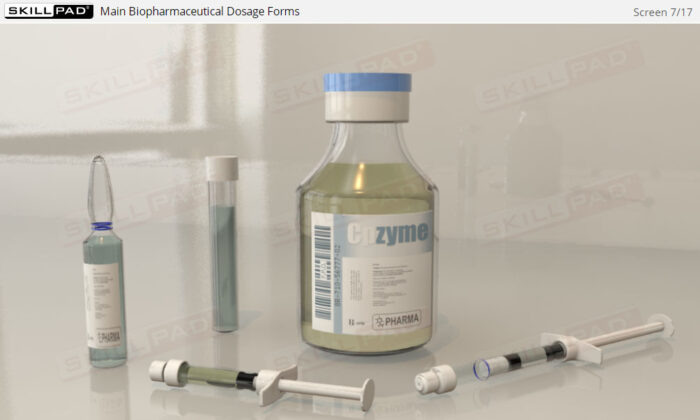
- Describe the main biopharmaceutical dosage forms.
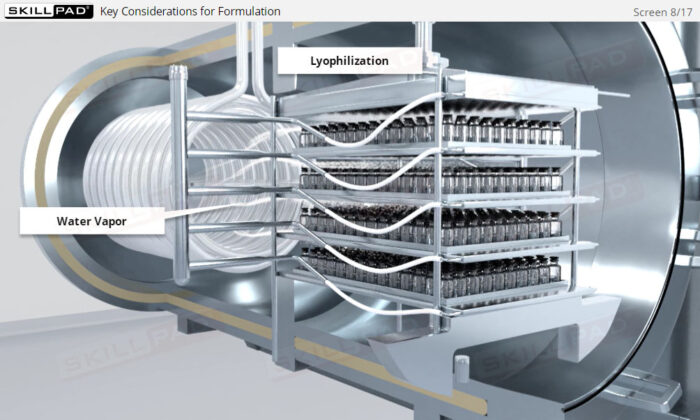
- Explain the key considerations for formulation: safety, stability, purity, effectiveness.
- Distinguish between the purpose of APIs and excipients in formulation.
- Describe the function of different excipient types.
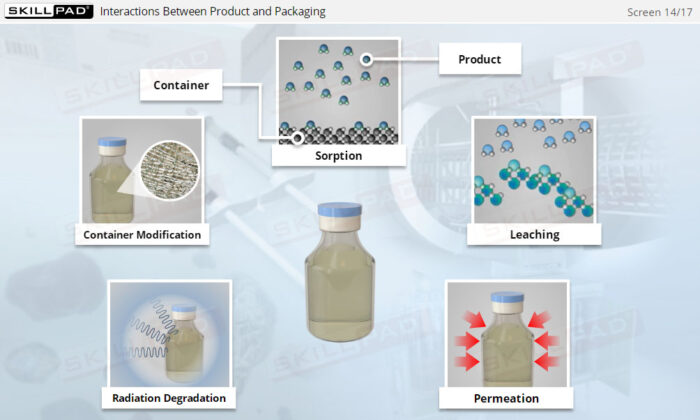
- Explain the various ways in which products may interact with packaging.
- Describe the factors involved in selecting packaging types.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen