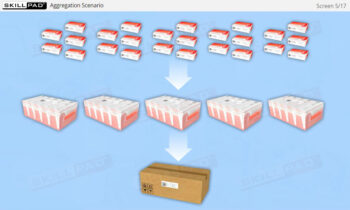
Four Level Serialization Structure
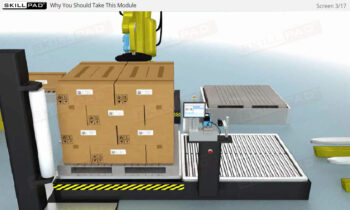
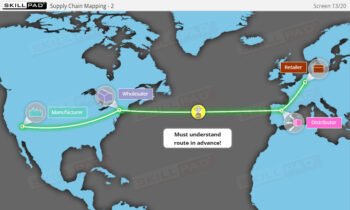
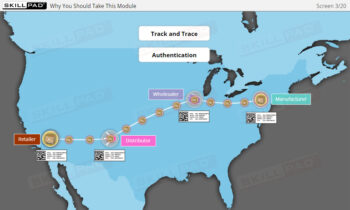
Serialization and product tracking are essential tools in combating counterfeit drugs and ensuring patient safety within the pharmaceutical and biologics industries. This module introduces the concepts of serialization and product tracking, focusing on their regulatory framework, implementation strategies, and the technologies used to maintain traceability. It covers the importance of “Track and Trace” and “Authentication,” the role of packaging lines, and the stages involved in rolling out a serialization program. Key challenges, including equipment integration, data integrity, and international standards, are also addressed to help users navigate the complexities of implementing these systems.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
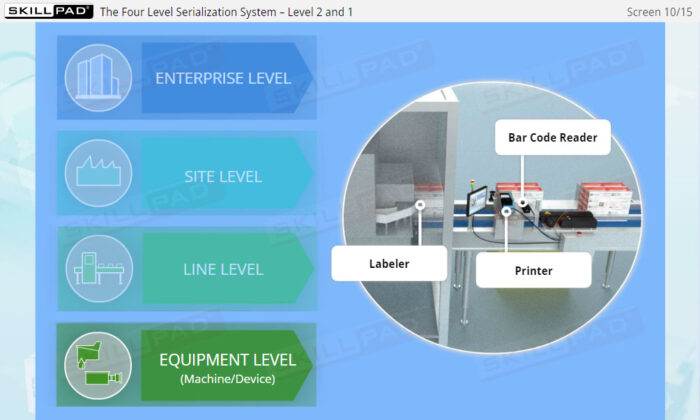
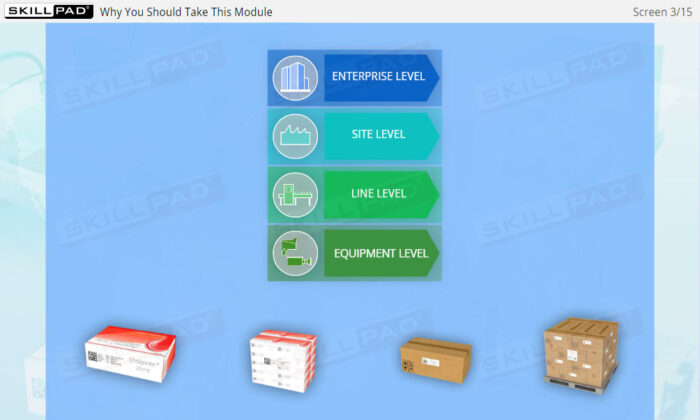
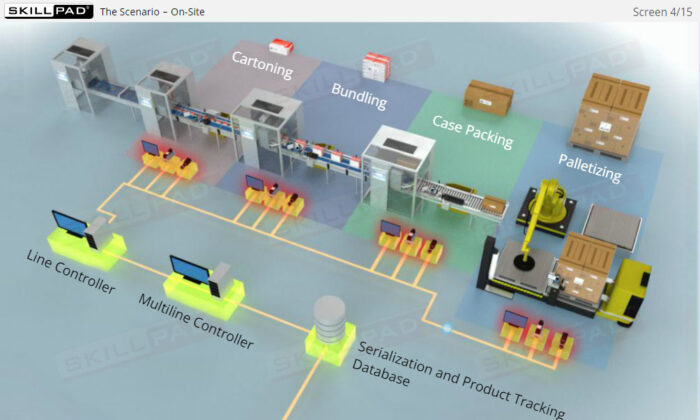
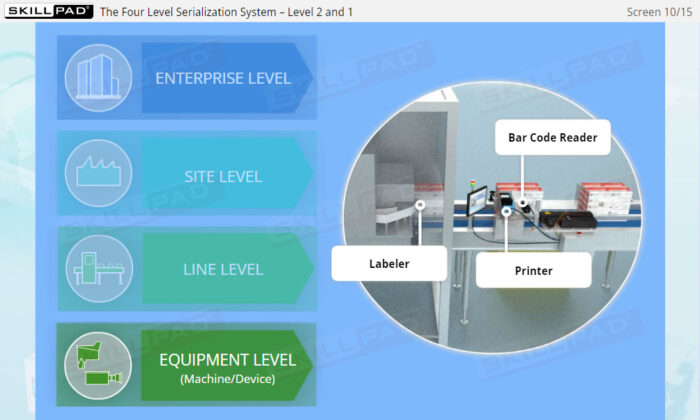
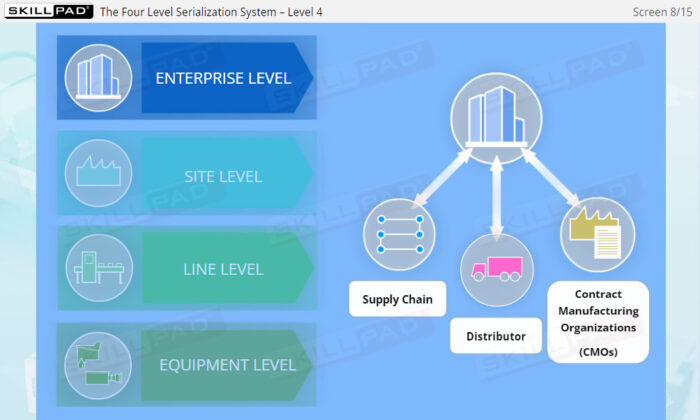
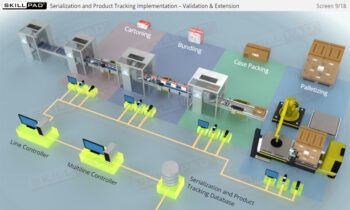
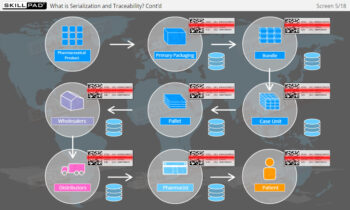
- Gain a Comprehensive Overview: Develop a clear understanding of the four-level serialization corporate structure, including the enterprise, site, packaging line, and machine/device levels.
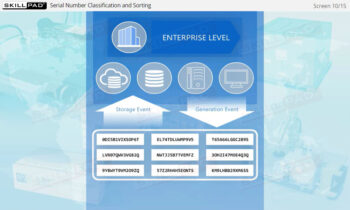
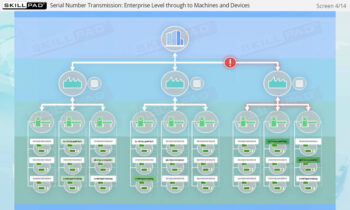
- Enhance Technical Knowledge: Understand how serial numbers are generated, stored, transmitted, and applied to packaging formats, ensuring product traceability and integrity throughout the supply chain.
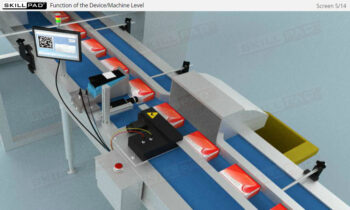
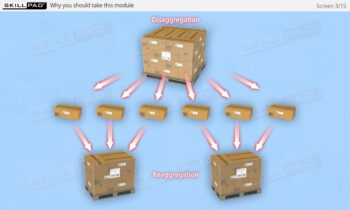
- Improve Practical Skills: Learn the IT functions associated with serialization at each level and how they support operations such as disaggregation, re-aggregation, and regulatory compliance.
- Strengthen Compliance Competence: Equip yourself with the knowledge to ensure serialization processes meet regulatory requirements, including traceability obligations under laws like the Drug Supply Chain Security Act (DSCSA).
- Reinforce Learning Through Interactivity:
Apply your learning through knowledge checks and explorations that reinforce understanding of the serialization process and its implementation.
Learning Objectives
- Describe the four-level serialization corporate structure.
- Explain what is meant by ‘top-down’ generation and transmission of serial numbers.
- Describe the IT functions associated with each of the four levels of the serialization corporate structure.
Keywords
- Serialization
- Track and Trace
- Four-Level Corporate Structure
- Serial Number Generation
- Regulatory Compliance
- Drug Supply Chain Security Act (DSCSA)
- Traceability
- Enterprise Level Serialization
- Site Level Serialization
- Packaging Formats
- IT Infrastructure
- Product Identification
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen