Freeze Drying in Biopharmaceutical Manufacturing
Describes freeze drying (lyophilization), its use in biopharmaceutical manufacturing, the structure of a freeze dryer and the freeze drying process, including critical parameters, cycle phases, process monitoring and control.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
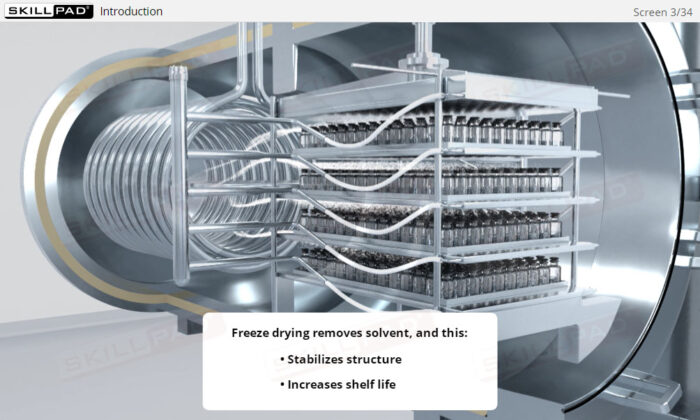
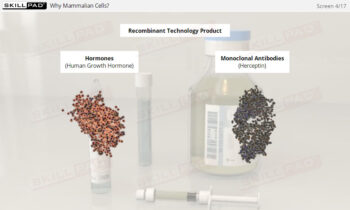
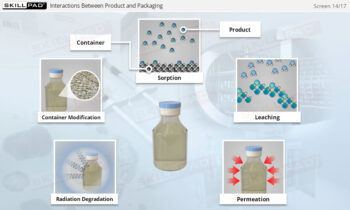
- Gain a comprehensive understanding of freeze drying, its purpose in biopharmaceutical manufacturing, and its significance in preserving the biological activity and therapeutic effects of heat-sensitive products.
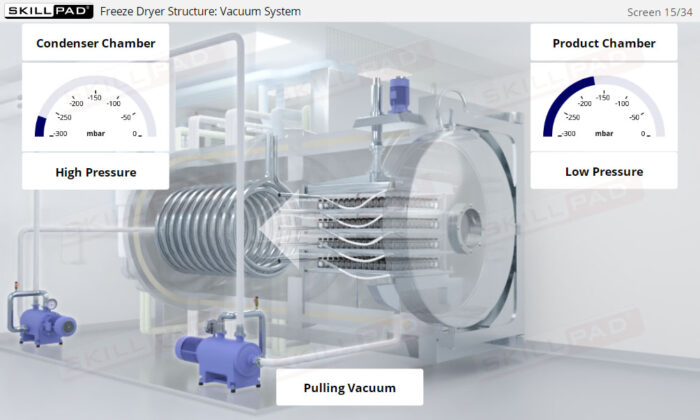
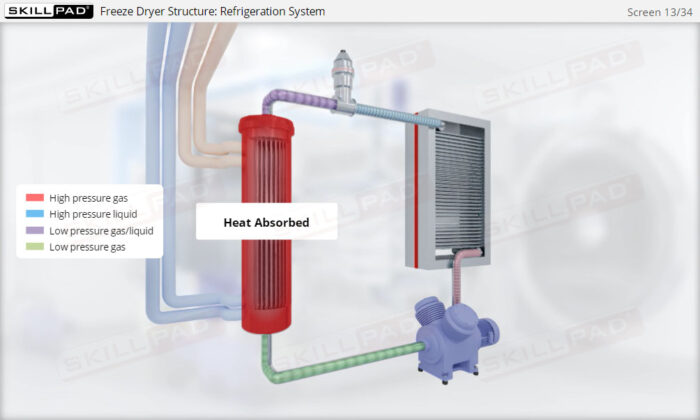
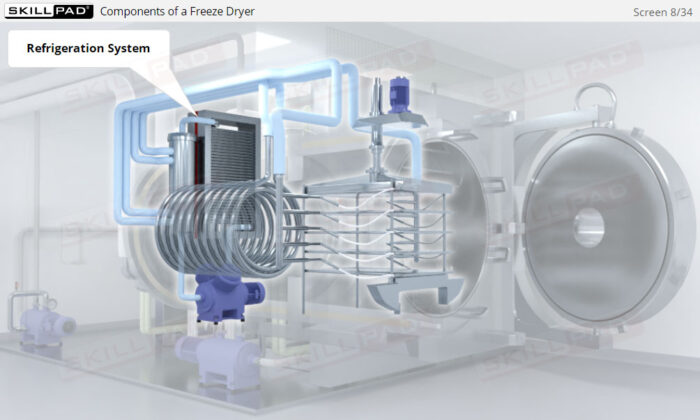
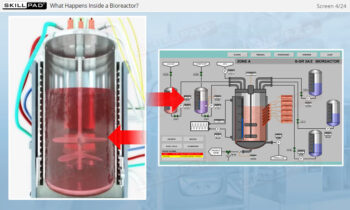
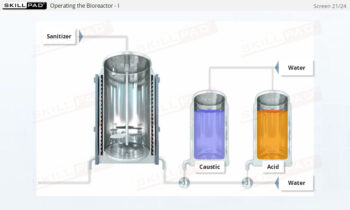
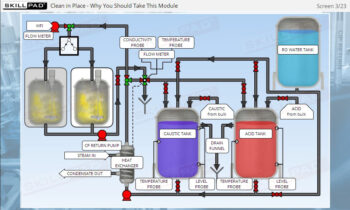
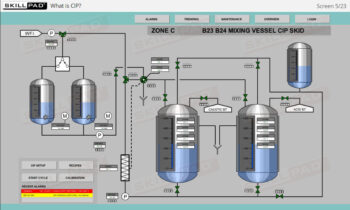
- Learn about the key components of a freeze dryer and their functions and deepen your understanding of the refrigeration and vacuum systems that support the freeze-drying process.
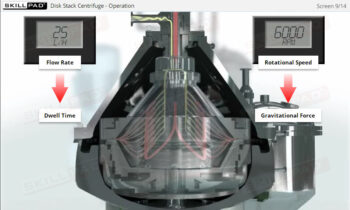
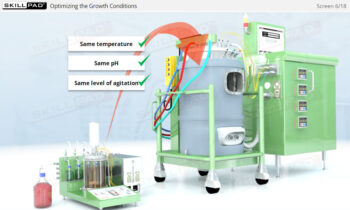
- Understand the phases of the freeze-drying cycle and how critical parameters like pressure, temperature, and time are controlled to achieve optimal drying and product stability.
Learning Objectives
- Describe the advantages and disadvantages of freeze drying.
- Describe the basic structure of a freeze dryer.
- Describe the brief history of freeze drying.
- Explain how both vacuum and refrigeration are used in freeze drying.
- Explain what is meant by freeze drying.
- Explain why freeze drying is used in the manufacture of biopharmaceuticals.
- List and describe the three phases of the freeze drying process.
- List and explain the critical freeze drying process parameters that must be monitored and controlled.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen