Fundamentals of Process Validation
An overview of process validation in regulated industries, such as pharmaceuticals, biopharmaceuticals, active pharmaceutical ingredients, and medical devices. This module explains the essential principles and regulatory requirements of process validation, highlighting its critical role in ensuring product quality, safety, and effectiveness. Learners will explore key concepts such as the Validation Life Cycle, Validation Master Plan, and risk-based approaches, alongside the distinct qualification stages (DQ, IQ, OQ, PQ) that demonstrate control over manufacturing processes and equipment. The module also covers tools such as the traceability matrix, change control, and revalidation strategies that help maintain a validated state.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Learn About Validation Principles: Understand the importance of validation within GMP-regulated environments and its impact on product quality, safety, and regulatory compliance.
- Understand Validation Categories: Learn to differentiate between the five types of validation and their application across manufacturing facilities, supporting a well-rounded understanding of equipment, systems, and processes.
- Conduct Risk Assessments: Learn how to perform risk assessments to optimize validation efforts using methods such as FMEA to prioritize resources effectively.
- Understand the Validation Life Cycle: Gain knowledge of the stages of the Validation Life Cycle and the importance of planning and documentation through tools like the Validation Master Plan.
- Learn About Qualification Stages: Understand the DQ, IQ, OQ, and PQ stages and how they establish the reliability and regulatory readiness of equipment and processes.
- Apply Change Control and Revalidation: Learn how to maintain process integrity through robust change control procedures and understand when revalidation is necessary to uphold product standards.
- Use Traceability Matrices: Learn how to track requirements from conception to execution, ensuring that all validation activities meet initial specifications and are well-documented.
Learning Objectives
- Explain the term ‘validation’ and why it must be performed in regulated industries.
- List the five established validation categories.
- Explain the risk assessment approach to validation.
- Explain the purpose of a Validation Master Plan (VMP) and describe its contents.
- Explain the concept of the Validation Life Cycle.
- Describe the different stages of the Validation Life Cycle.
- Explain ‘traceability matrix’ and describe its use in validation.
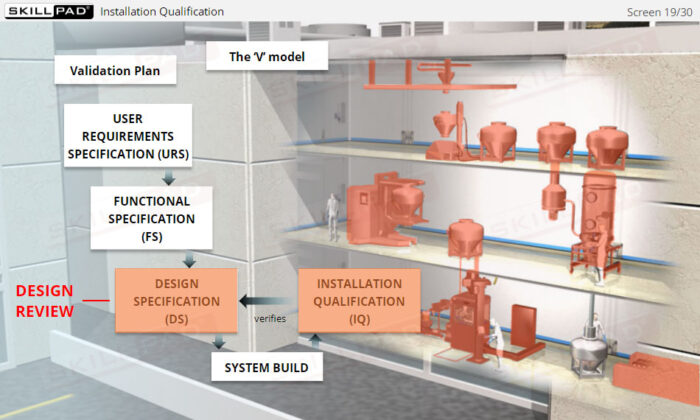
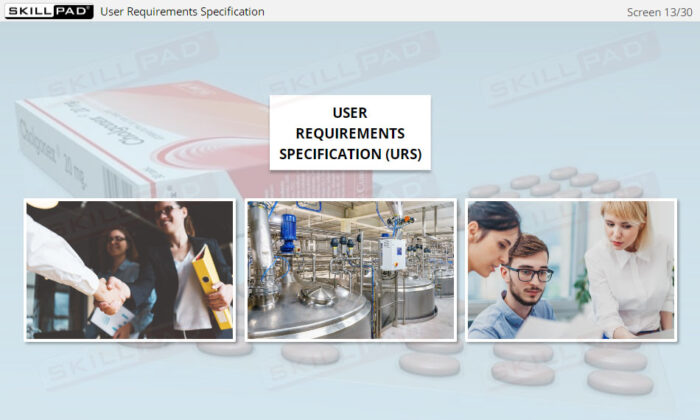
- Explain the terms Design Specification (DS), Functional Specification (FS), and User Requirements Specification (URS).
- Explain the purpose of process validation.
- Describe each of the four qualification stages: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
- Explain how the qualification stages relate to the equipment specifications.
- Explain the concepts of change control, revalidation and decommissioning.
Keywords
- Analytical Validation
- Change Control
- cGMP Compliance
- Cleaning Validation
- Computerized Systems Validation
- Equipment Validation
- Failure Modes and Effects Analysis (FMEA)
- Process Validation
- Regulatory Compliance
- Revalidation
- Risk Assessment
- Traceability Matrix
- Qualification (DQ, IQ, OQ, PQ)
- Validation Life Cycle
- Validation Master Plan (VMP)
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen