GCP Essential Documents: Investigators Brochure & Study Protocol
An overview of two key documents used in a clinical trial – the Investigator’s Brochure and the Study Protocol. The purpose of each document is described along with recommended sections for inclusion according to ICH E6 guidelines.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Gain a Clear Understanding of Key Clinical Trial Documents: Develop a comprehensive understanding of the Investigator’s Brochure (IB) and Study Protocol, including their purposes, essential components, and alignment with ICH E6 guidelines.
- Identify Recommended Sections for Essential Documents: Recognize the critical sections to include in the Investigator’s Brochure and Study Protocol to support effective trial management and regulatory compliance.
- Enhance Communication Through Documentation: Explore how clear and accurate documentation improves the communication of complex trial information to diverse audiences, including non-scientific stakeholders.
- Strengthen Compliance and Trial Integrity: Learn best practices for documentation to meet regulatory requirements, minimize errors, and uphold the integrity of clinical trials.
Learning Objectives
- Explain why documentation is essential in clinical studies.
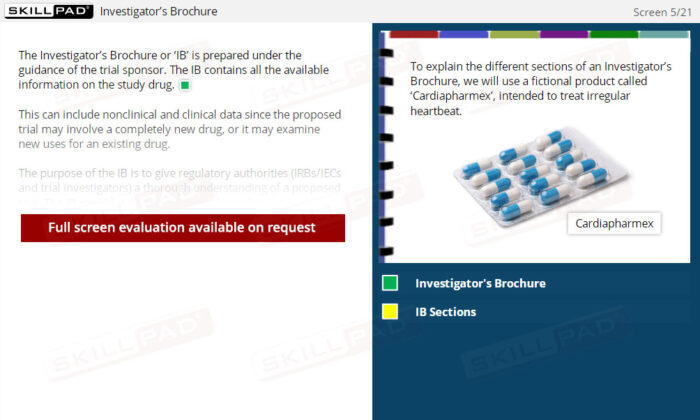
- Explain the purpose of an Investigator’s Brochure in a clinical trial.
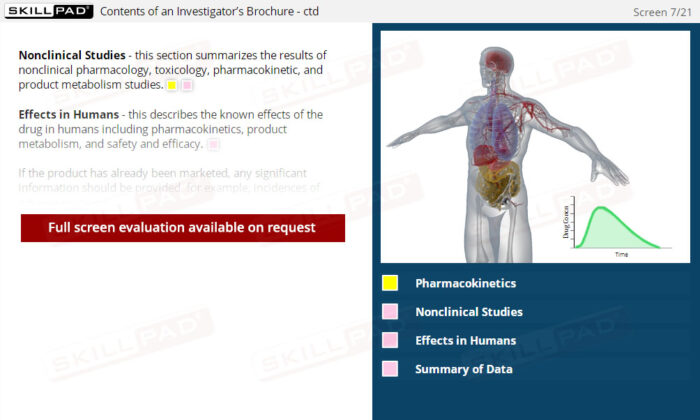
- List the ICH recommended sections of an Investigator’s Brochure.
- Explain the purpose of a Study Protocol in a clinical trial.
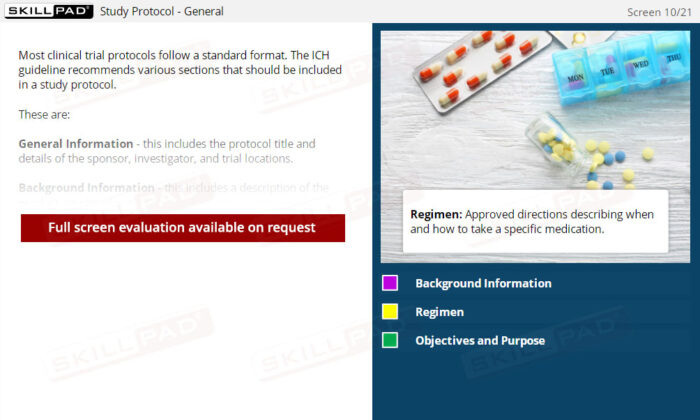
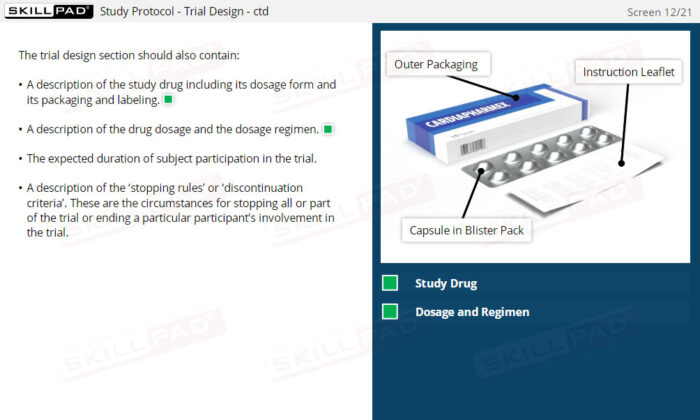
- List the ICH recommended sections of a Study Protocol.
- Explain why clarity and conciseness are important in a Study Protocol.
- Define ‘Protocol Amendment’ and why it might be necessary.
Keywords
- Clinical Trials
- Clinical Trial Protocol
- Good Clinical Practice (GCP)
- Human Subjects
- ICH GCP
- Independent Ethics Committee (IEC)
- Institutional Review Board (IRB)
- Informed Consent
- Regulatory Authorities
- Sponsors
- Trial Safety and Welfare
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen