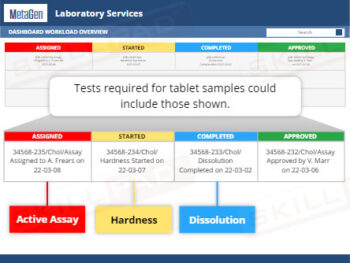
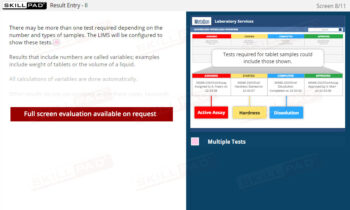
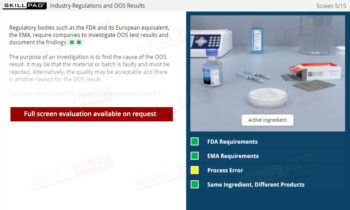
GLP – Working in the Laboratory
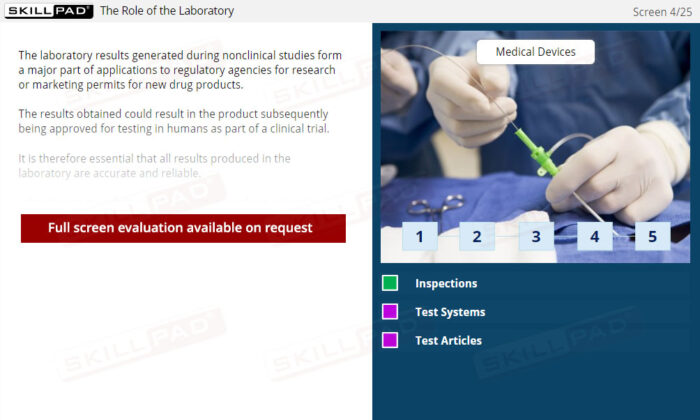
Gain a comprehensive understanding of Good Laboratory Practice (GLP) and its critical role in nonclinical studies. This module outlines the purpose of nonclinical research and the necessity of adhering to GLP regulations in the research laboratory. Explore essential procedures including the correct completion of laboratory records, sample receipt and storage protocols, preparation steps for testing, data management, and the approval process for test results. Emphasizing accuracy and reliability, this module provides the knowledge required to maintain compliance with GLP standards, ultimately ensuring the safety and effectiveness of drug products before clinical trials.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- In-depth Understanding of Nonclinical Studies: Acquire a foundational understanding of nonclinical studies, their purposes, and the significance of GLP in ensuring that laboratory results are reliable and accurate.
- Mastery of GLP Requirements: Learn the essential GLP regulations that govern laboratory practices, enabling you to contribute effectively to the safety and compliance of drug development processes.
- Correct Record-Keeping Skills: Develop proficiency in completing laboratory records accurately, adhering to GLP standards to maintain integrity and accountability in data management.
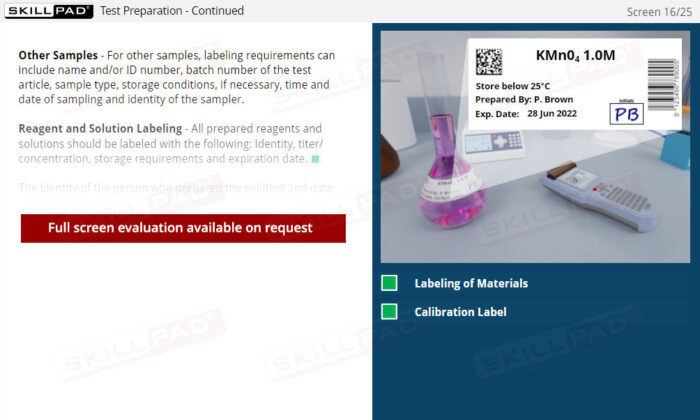
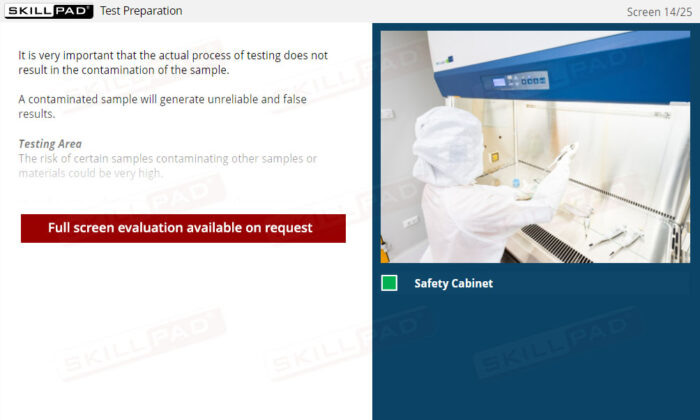
- Expertise in Sample Management: Gain insights into proper sample receipt and storage techniques, understanding the impact of storage conditions on the validity of test results.
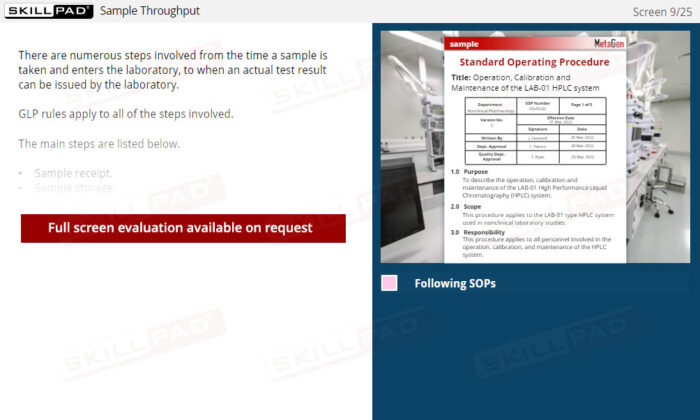
- Preparation and Testing Protocol Knowledge: Equip yourself with the steps necessary for preparation and testing in the laboratory, enhancing your ability to follow standard operating procedures (SOPs) correctly and consistently.
- Data Handling and Approval Processes: Understand the lifecycle of data generated from sample analyses, including how to properly handle, check, and approve test results in alignment with GLP requirements.
Learning Objectives
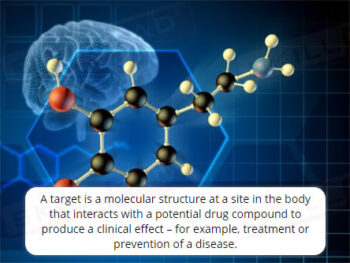
- Explain the purpose of nonclinical studies.
- Explain what is meant by ‘Good Laboratory Practice’ (GLP).
- Complete laboratory records correctly.
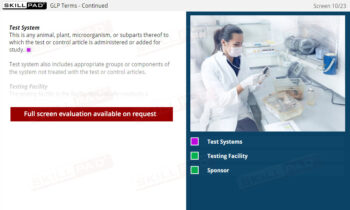
- Describe what is involved in sample receipt and storage.
- Outline the steps that the analyst must take to prepare for testing in terms of solution and equipment preparation.
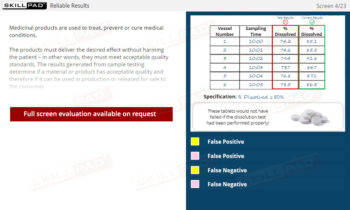
- Describe what is done with data generated through sample analysis.
- Explain how sample test results are approved in the laboratory.
Keywords
- Analyst responsibilities
- Calibration
- Drug development
- GLP regulations
- Good Laboratory Practice (GLP)
- Nonclinical studies
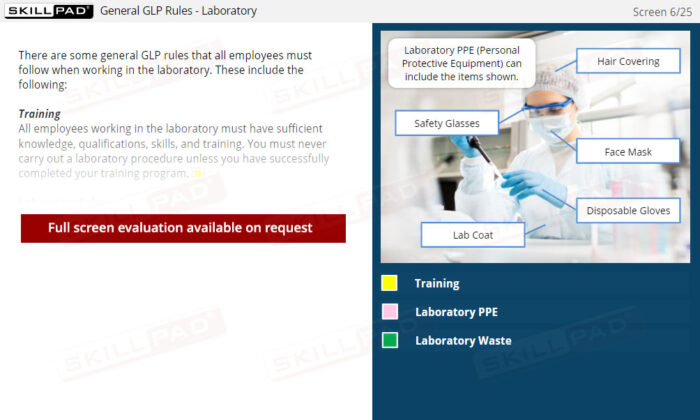
- Personal Protective Equipment (PPE)
- Record-keeping
- Standard Operating Procedures (SOP)
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen