GMP for APIs
Explores the foundations of Good Manufacturing Practices (GMP) in Active Pharmaceutical Ingredient (API) manufacturing, covering its historical development, key regulatory milestones, and the essential characteristics of GMP-compliant APIs. Learners will examine the areas of API production where GMP applies, including warehousing, quality control, and packaging. The module also highlights the importance of adhering to GMP to ensure the safety, purity, and effectiveness of APIs, and introduces the concept of current GMP (cGMP), reflecting the latest industry standards.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the History of Pharmaceutical Regulation: Understand the historical context of pharmaceutical regulation and the key events that led to the development of GMP standards.
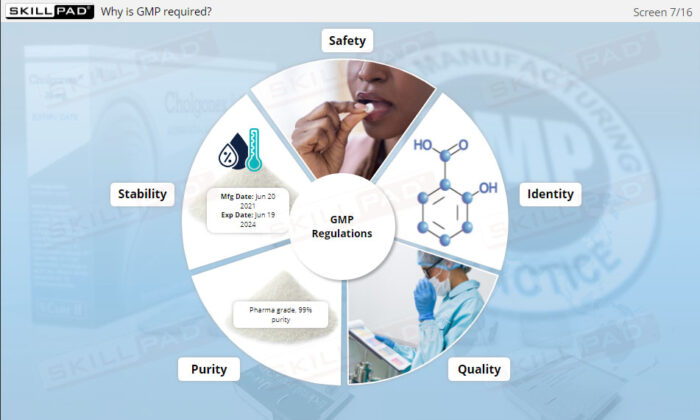
- Identify GMP Compliance Requirements for APIs: Learn the essential characteristics of GMP-compliant APIs, including safety, identity, quality, purity, and stability.
- Recognize Where GMP Applies in API Manufacturing: Explore the areas of API manufacturing where GMP is critical, such as warehousing, quality control, production, and packaging.
- Understand the Importance of GMP: Learn why following GMP is essential for ensuring the safety, purity, and effectiveness of medicines, and how it helps protect both consumers and manufacturers.
- Gain Insight into cGMP Standards: Understand the concept of current GMP (cGMP) and why staying up-to-date with industry advancements and regulatory changes is important.
Learning Objectives
- Explain the historical reasons for how the pharmaceutical industry came to be regulated.
- List the essential characteristics of a GMP-compliant active pharmaceutical ingredient.
- List the areas of API manufacturing where GMP applies.
- Describe the benefits of manufacturing API products according to GMP.
- Explain what is meant by ‘cGMP’.
Keywords
- Active Pharmaceutical Ingredient (API)
- cGMP
- Contamination Prevention
- Food and Drug Act
- GMP Compliance
- GMP Regulations
- GMP Requirements
- Good Manufacturing Practices
- Identity
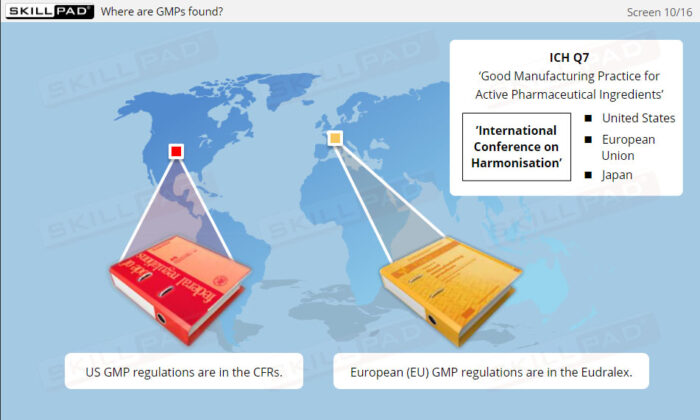
- ICH Q7
- Ingredient Safety
- Labeling
- Manufacturing Standards
- Pharmaceutical Industry
- Pharmaceutical Safety
- Production Quality
- Purity
- Quality Control
- Stability
- Sulfanilamide Elixir
- Thalidomide
- Water Quality
- Warehousing
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen