GMP for Finished Dose Forms
A foundational exploration of Good Manufacturing Practices (GMP) in the pharmaceutical industry, focusing on the historical events that led to the establishment of these critical regulations. The module outlines the essential attributes of GMP-compliant drug products—such as safety, identity, strength, quality, purity, and stability—and explains the application of GMP across various stages of pharmaceutical manufacturing. Additionally, it highlights the regulatory requirements for finished dose forms and emphasizes the importance of adhering to current Good Manufacturing Practices (cGMP) to ensure product safety and quality for consumers.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
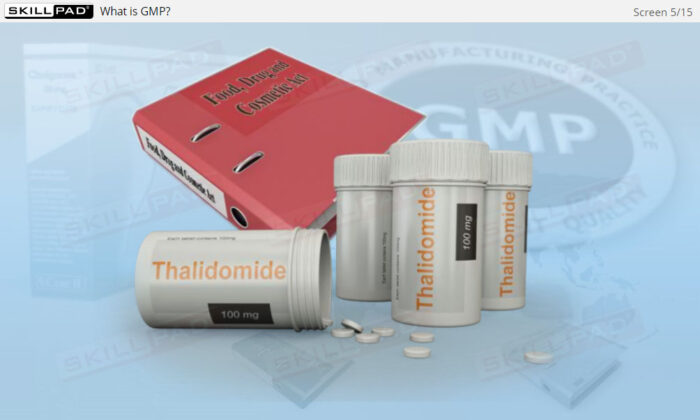
- Learn About the Evolution of Pharmaceutical Regulations: Understand the historical events that led to the regulation of drug manufacturing, reinforcing the role of GMP in protecting public health.
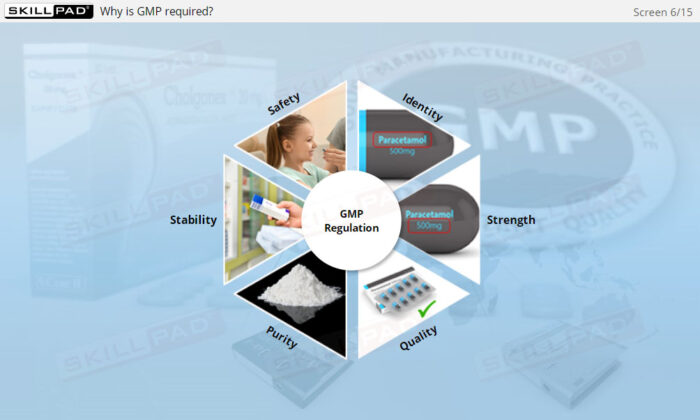
- Understand Drug Product Attributes: Gain foundational knowledge of the key characteristics that define a GMP-compliant drug product, supporting an understanding of product safety and integrity standards.
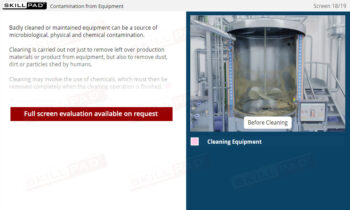
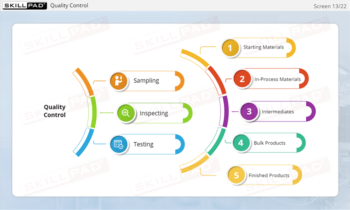
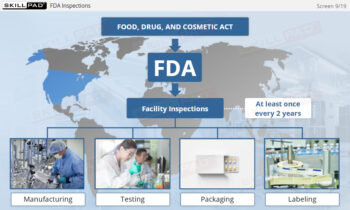
- Explore GMP Application Areas: Learn how GMP applies across various pharmaceutical operations, such as warehousing, laboratories, production, packaging, and labeling, to support quality assurance practices.
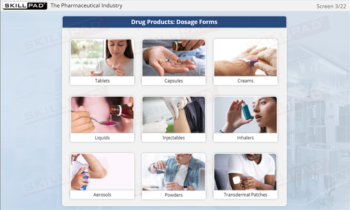
- Understand Regulatory Compliance for Finished Dose Forms: Build a foundational understanding of U.S. and European GMP regulations for finished dose manufacturing, supporting compliance in global markets.
- Recognize the Importance of cGMP: Learn why ‘current’ GMP (cGMP) emphasizes the need for continuous updates to align with technological advancements and industry practices.
Learning Objectives
- Explain the historical reasons for how the pharmaceutical industry came to be regulated.
- Explain what is meant by cGMPs.
- List the essential characteristics of a GMP-compliant drug product.
- List the areas of pharmaceutical manufacturing where cGMPs apply.
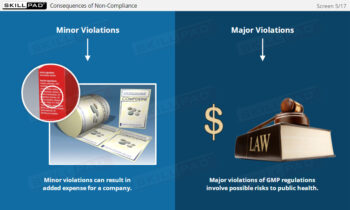
- Describe the benefits of manufacturing drug products according to cGMPs.
Keywords
- cGMP (current Good Manufacturing Practices)
- Contamination Prevention
- Drug Product Safety
- Eudralex
- FDA Regulations
- Finished Dose Forms
- Good Manufacturing Practices (GMP)
- Quality Assurance
- Regulatory Standards
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen