GMP for Microbiology
Explore the critical application of Good Manufacturing Practices (GMP) within a microbiology laboratory setting. This comprehensive module covers essential topics such as the reception, storage, and labeling of samples; the preparation and aseptic techniques necessary for accurate microbial testing; and the critical steps to prevent contamination and ensure reliable results. Emphasis is placed on understanding and adhering to GMP rules for laboratory work, including the proper use of PPE, handling equipment like pipettes, and the methods for addressing laboratory spills to uphold safety and product integrity.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
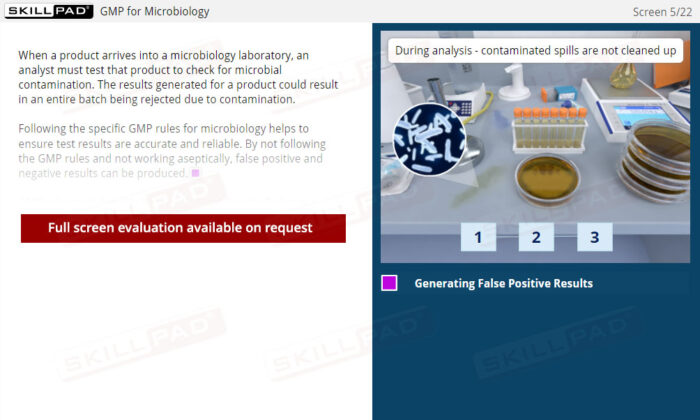
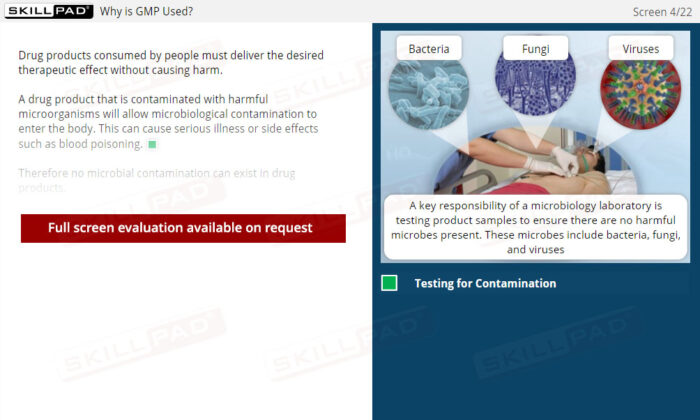
- Deep Understanding of GMP Principles for Microbiology: Learn the core regulations and best practices for working in microbiology labs to ensure compliance and accuracy in microbial testing.
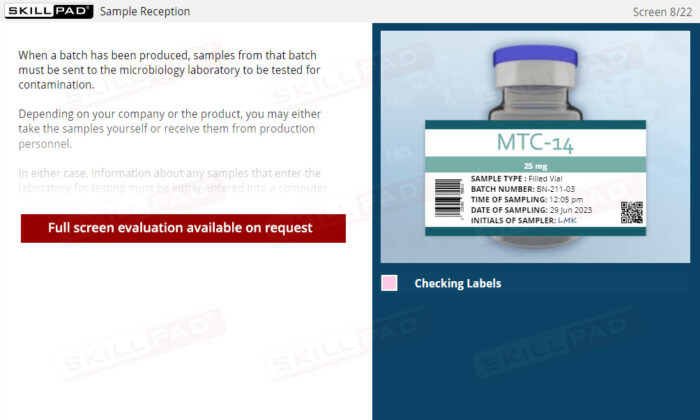
- Master Sample Handling and Storage: Gain proficiency in receiving, logging, and storing samples correctly to avoid cross-contamination and maintain the validity of results.
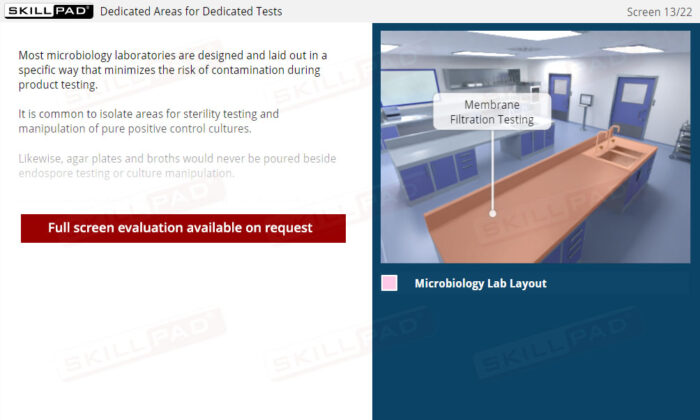
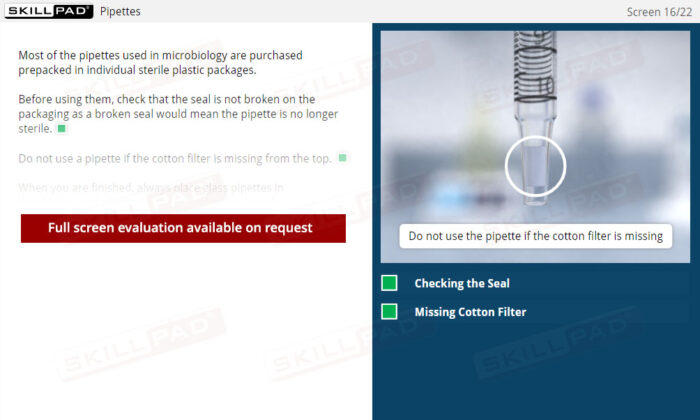
- Aseptic Technique and Work Area Preparation: Develop essential skills in maintaining sterile work environments, including the use of dedicated areas, proper bench sterilization, and safety cabinet procedures to prevent false test outcomes.
- Labeling Procedures: Understand the importance of precise labeling for all laboratory materials, from agar plates and broths to solutions and waste, ensuring traceability and minimizing errors.
- Efficient Spill Management: Techniques for safely and effectively managing spills, minimizing risk to personnel and maintaining laboratory integrity.