GMP Goals
Exploration of the critical role GMP plays in ensuring the safety, quality, and efficacy of pharmaceutical products. It explains the purpose of GMP regulations, their application in manufacturing facilities, and the personal and organizational responsibilities required to maintain compliance.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the Purpose of GMP Regulations: Gain a clear understanding of why GMP regulations are essential for ensuring safe, effective, and high-quality pharmaceutical product.
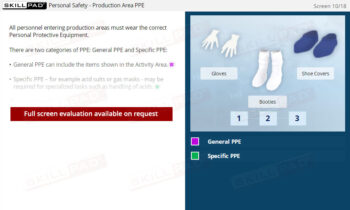
- Appreciate Personal Responsibility in GMP Compliance: Learn how individual actions, such as wearing appropriate PPE and following written procedures, directly impact the success of a GMP system.
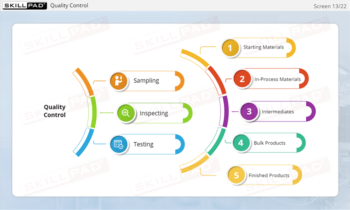
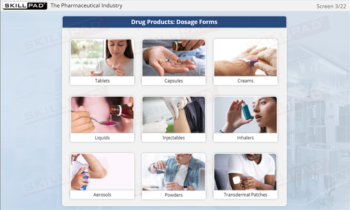
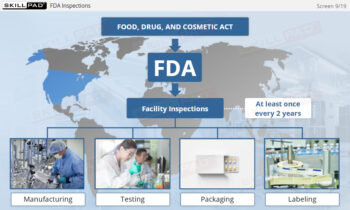
- Identify GMP-Applicable Areas in Manufacturing: Explore the various facility areas—such as personnel, production, quality control laboratories, and packaging—where GMP principles must be applied.
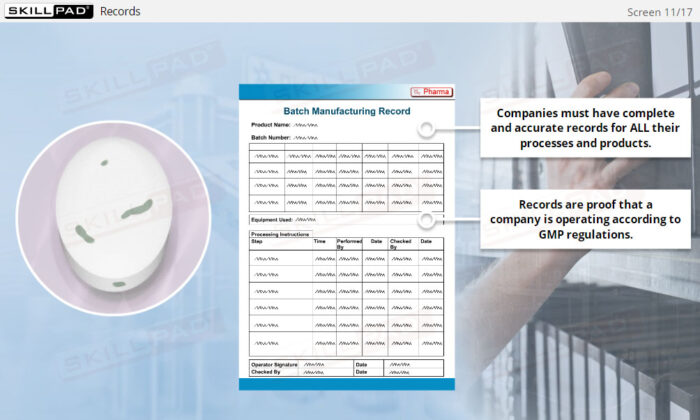
- Recognize the Importance of Following GMP Procedures: Understand the importance of consistent adherence to Standard Operating Procedures (SOPs) and learn best practices for documenting changes and maintaining accurate records.
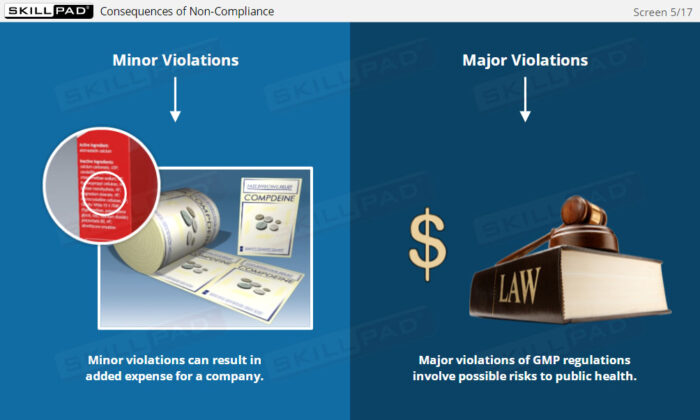
- Gain Awareness of Consequences of Non-Compliance: Learn about the potential legal, financial, and reputational risks that noncompliance poses to both companies and individuals.
Learning Objectives
- Describe the purpose of GMP Regulations in pharmaceutical manufacturing.
- Explain why GMP Regulations must be followed in a pharmaceutical manufacturing facility.
- List the areas in a pharmaceutical manufacturing facility where GMP regulations apply.
- Explain how personal responsibility affects the success of a GMP system of working.
- Explain why procedures and records are an essential component of GMP.
Keywords
- GMP Regulations
- Pharmaceutical Quality
- Regulatory Compliance
- Personal Responsibility
- GMP System of Working
- Procedures
- Records
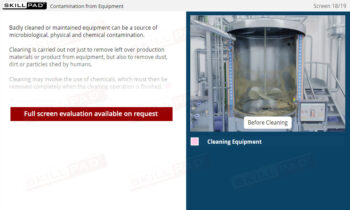
- Equipment Maintenance
- Consumer Protection
- Manufacturing Standards
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen