GMP Inspection Readiness – Interacting with the Inspector
Essential knowledge and techniques for effective interaction with inspectors during a GMP inspection. This module covers the inspection process, typical types of questions asked, and appropriate behaviors and responses for personnel involved, ensuring a positive inspection outcome.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Strategic
Description
- Gain a Thorough Understanding of GMP Inspections: Learn about the purpose of GMP inspections, who is involved, and what inspectors typically focus on, so you can approach inspections with confidence.
- Master Communication Skills for Inspection Scenarios: Recognize and respond to various types of inspector questions, enhancing your ability to engage effectively and professionally during inspections.
- Enhance Professionalism and Preparedness: Develop skills in maintaining appropriate behaviors and following best practices when interacting with inspectors, contributing to a smooth and successful inspection process and outcome.
- Apply Practical Knowledge in Realistic Scenarios: Benefit from scenario-based exercises that simulate inspection situations, allowing practice and reinforcement of understanding in a safe learning environment.
Learning Objectives
- Describe the purpose of a GMP Inspection.
- Describe what typically happens in a GMP Inspection and who is involved.
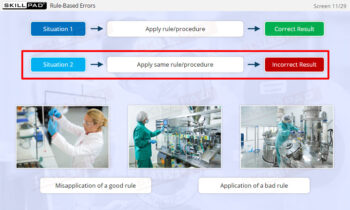
- Recognize the different types of questions an Inspector can ask in a GMP Inspection.
- Respond appropriately to question typically asked in a GMP Inspection.
- Demonstrate appropriate behaviours during a GMP Inspection.
Keywords
- Compliance
- GMP Inspection Readiness
- Inspection Behavior
- Inspector Interaction
- Inspection Preparation
- Inspection Scenarios
- Quality Systems
- Regulatory Compliance
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen