GxP Computerized Systems Validation
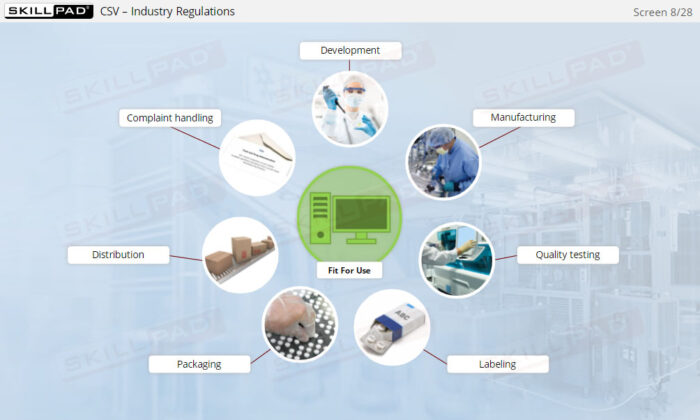
An overview of the critical concepts and key requirements of Computerized Systems Validation (CSV) in regulated life sciences industries, including pharmaceuticals, biologics, and medical devices. Delve into essential aspects like system lifecycle, risk assessment, and regulatory requirements such as FDA’s 21 CFR Part 11. The module covers how to ensure computerized systems remain ‘fit for use’ to uphold product quality, patient safety, and data integrity.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the Role of Computerized Systems in Regulated Environments: Develop a foundational understanding of computerized systems, their role, and their impact in GxP-regulated environments.
- Ensure Compliance with Industry Regulations: Learn practical methods for validating systems in accordance with key regulations such as 21 CFR Part 11 and understand the consequences of non-compliance, including regulatory action and potential penalties.
- Evaluate Risks and Apply Lifecycle Approaches: Learn to assess risks associated with computerized systems and integrate validation throughout the system’s lifecycle to maintain compliance and operational integrity.
- Implement and Document CSV Effectively: Gain insights into planning, specifying, configuring, and verifying computerized systems while recognizing the importance of thorough documentation to ensure system reliability and regulatory readiness.
Learning Objectives
- Define ‘computerized system’.
- Explain the purpose of computerized system validation.
- Explain the purpose of the 21 CFR Part 11 regulation.
- Define ‘risk’ as it relates to computerized systems.
- Describe how software in computerized systems is categorized.
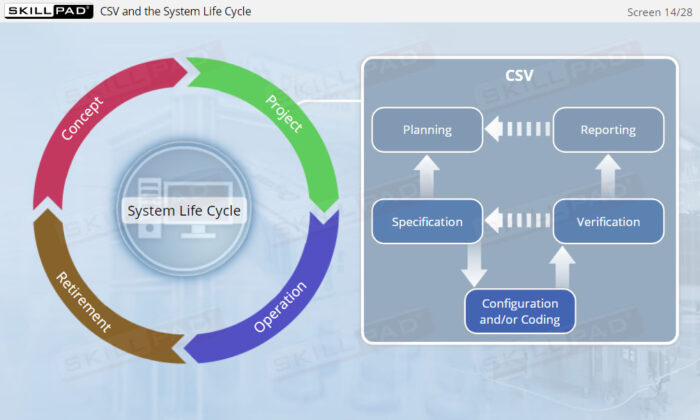
- Explain the purpose of incorporating CSV into the system life cycle approach.
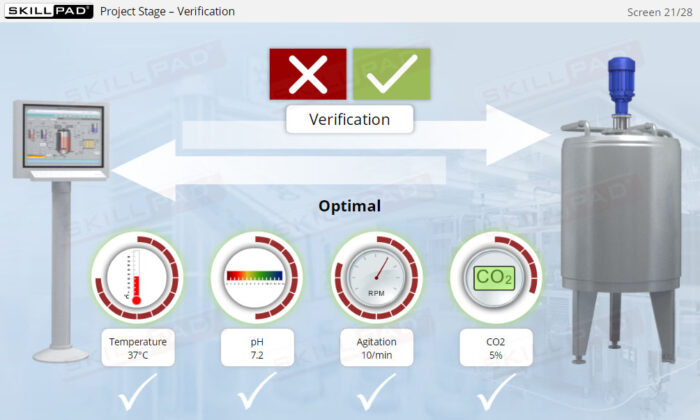
- Describe the activities involved in the project phase of CSV.
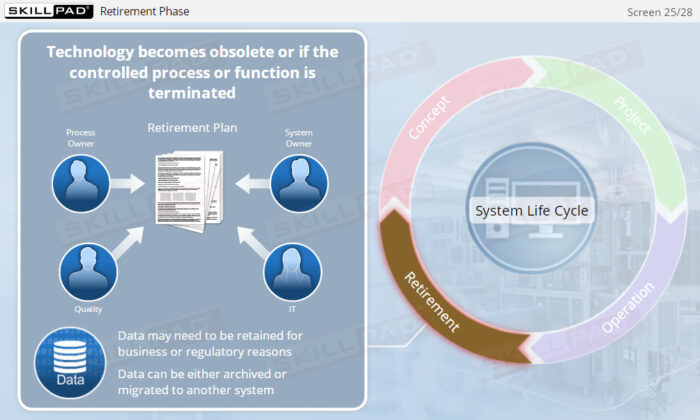
- Explain why risk management must be done throughout the operating phase of a computerized system.
Keywords
- Computerized Systems Validation (CSV)
- Data integrity
- FDA 21 CFR Part 11
- Good Clinical Practices (GCP)
- Good Laboratory Practices (GLP)
- Good Manufacturing Practices (GMP)
- GxP regulations
- Risk assessment
- System lifecycle
- Validation documentation
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen