GxP Good Documentation Practices – Applications
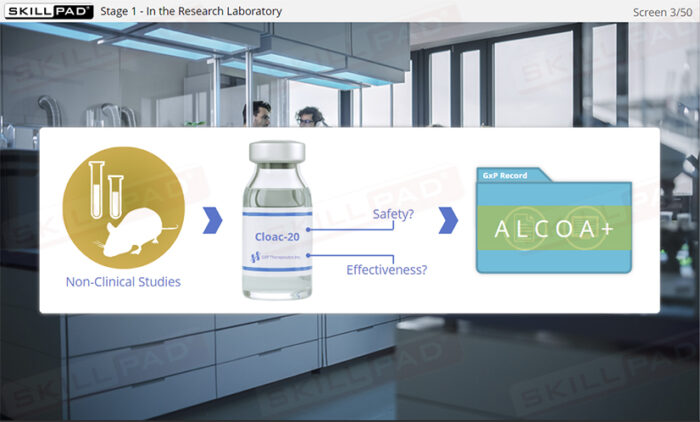
The critical importance of Good Documentation Practices (GDocP) in GxP-regulated industries, focusing on the application of ALCOA+ principles to ensure data integrity across all stages of product development. The module follows the lifecycle of a fictional product, Cloac-20, from research to commercial manufacturing, offering practical scenarios and best practices for both paper and electronic records.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Strategic
Description
- Thorough Understanding of Good Documentation Practices: Gain a detailed understanding of Good Documentation Practices (GDocP) and the ALCOA+ principles, essential for maintaining data integrity in GxP-regulated records.
- Application of GDocP to Real-World Scenarios: Learn how to effectively apply GDocP principles to both paper and electronic records, ensuring accurate and trustworthy data in real-world situations.
- Enhanced Decision-Making and Problem-Solving Skills: Strengthen your ability to address deviations and make informed corrections to GxP records through interactive and practical tasks.
Learning Objectives
- Explain the importance of Good Documentation Practices (GDocP) for data integrity in GxP-regulated industries.
- Identify the ALCOA+ principles and their significance in maintaining data integrity for GxP records.
- Distinguish between the core ALCOA principles and the additional “+” principles of ALCOA+.
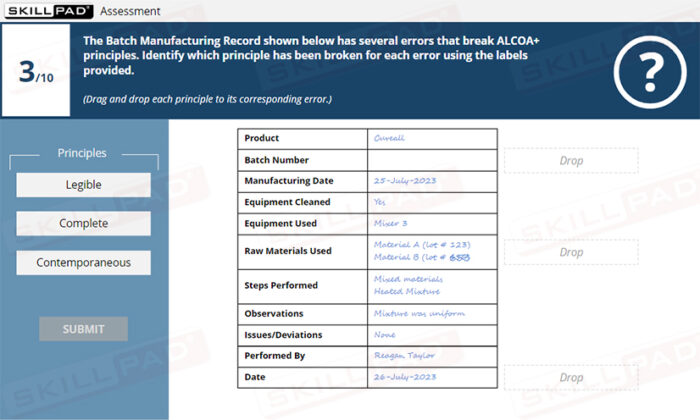
- Apply ALCOA+ principles to the completion of GxP paper and electronic records.
- Demonstrate best practices for correcting mistakes and making changes in GxP paper and electronic records.
- Demonstrate the ability to recognize and address potential deviations from ALCOA+ principles in recordkeeping processes.
Keywords
- ALCOA+ Principles
- Data Integrity
- Error Management
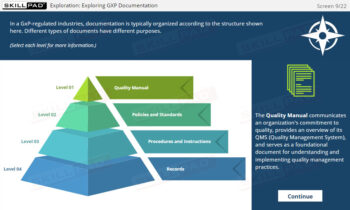
- Good Documentation Practices (GDocP)
- GxP Records
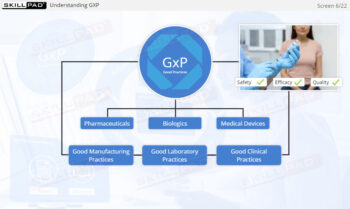
- GxP-Regulations
- Paper and Electronic Records
- Quality Assurance
- Regulatory Compliance
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen