GxP Good Documentation Practices – Principles
Essential principles of Good Documentation Practices (GDocP) for GxP-regulated industries, emphasizing data integrity and the ALCOA+ framework. The critical importance of accurate and reliable records in ensuring compliance with regulatory standards across various GxP sectors is reinforced throughout, while practical examples highlight key GDocP concepts, the role of signatures, and the potential consequences of non-compliance.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Strategic
Description
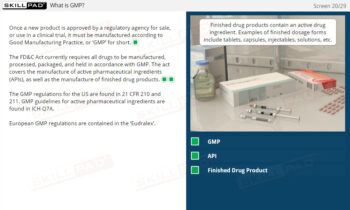
- Comprehensive Understanding of Good Documentation Practices: Gain a detailed insight into the critical importance of Good Documentation Practices (GDocP) in maintaining compliance with GxP regulations.
- Significance of Proper Documentation: Recognize how accurate and reliable documentation supports product safety, quality, and regulatory compliance in GxP-regulated industries.
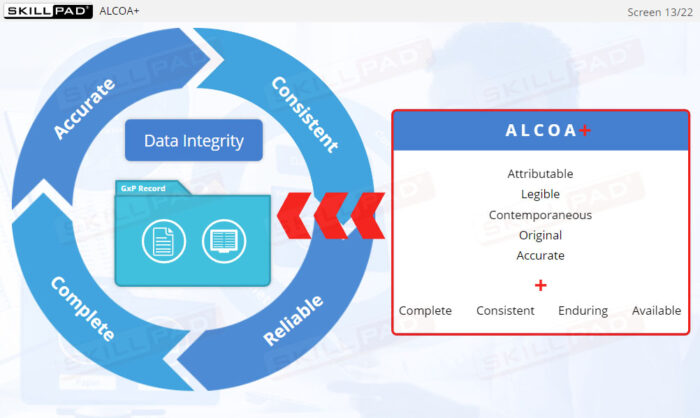
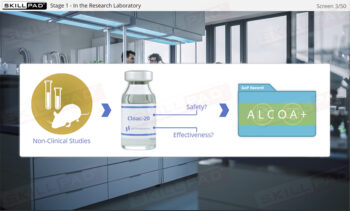
- Mastery of Data Integrity Principles: Learn the foundational principles of data integrity and how to apply ALCOA+ principles to create trustworthy and reliable records.
- Practical Recordkeeping Skills: Develop a hands-on understanding of recordkeeping requirements, including the role of signatures in ensuring accountability and preventing data manipulation.
Learning Objectives
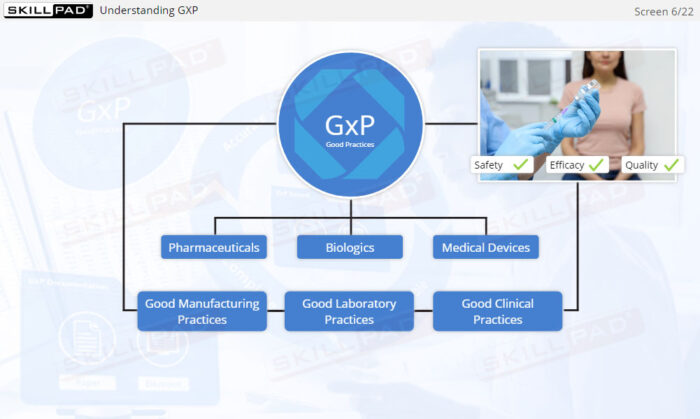
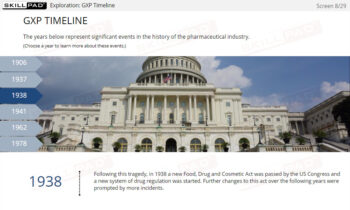
- Explain why Good Documentation Practices (GDocP) are essential in GxP-regulated industries.
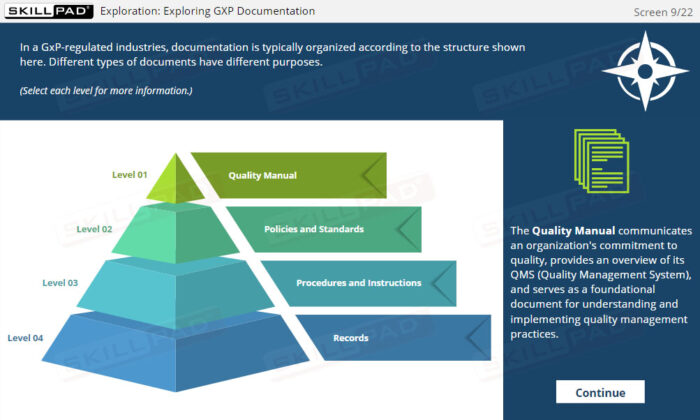
- Differentiate between directive documents and data collection documents.
- Define data integrity in the context of GxP records.
- Describe the relationship between ALCOA+ principles and the data integrity of GxP records.
- Recognize the potential consequences of not adhering to Good Documentation Practices.
- Explain the significance of signatures in GDocP for records.
Keywords
- ALCOA+ Principles
- Documentation
- Clinical Trials
- Data Accuracy and Reliability
- Data Integrity
- Good Documentation Practices (GDocP)
- GxP Records
- GxP Regulations
- Regulatory Compliance
- Quality Assurance
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen