Labeling
A guide to labeling practices in pharmaceutical and biotech manufacturing facilities, focusing on the identification and tracking of materials, products, and equipment. This module explains label requirements for various stages of production, the critical role of label accuracy in avoiding costly errors, and the secure handling of labels using a designated label cage. It also addresses safety labeling, equipment status indicators, and Good Labeling Practices essential for regulatory compliance and quality assurance.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
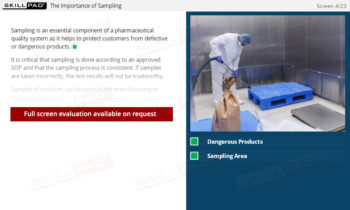
- Learn About the Importance of Labeling: Understand how accurate labeling prevents costly errors, ensures patient safety, and supports regulatory compliance in manufacturing environments.
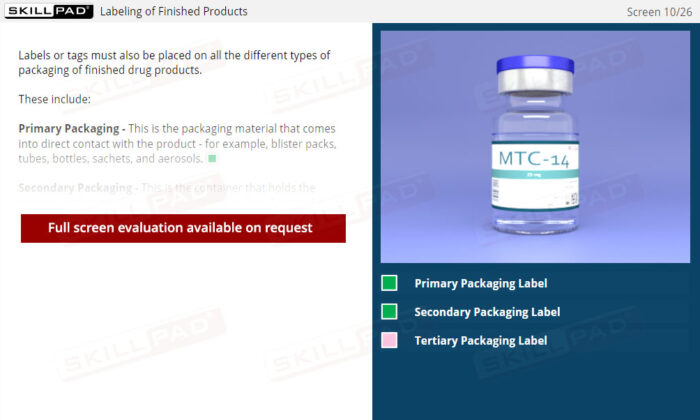
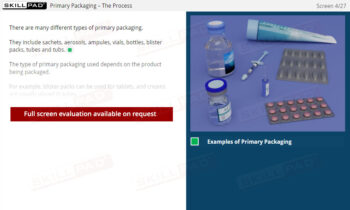
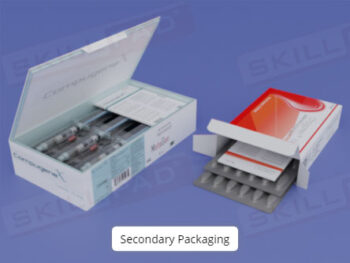
- Understand Labeling Requirements and Types: Learn about the different types of labels needed for raw materials, hazardous materials, equipment, and finished products, along with the information each must contain.
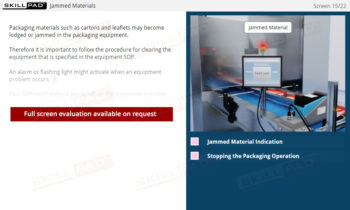
- Recognize Safety and Equipment Labeling Standards: Understand the importance of labeling hazardous materials, packaging, and equipment to prevent risks and ensure proper usage.
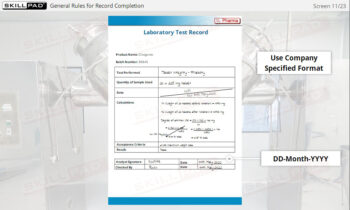
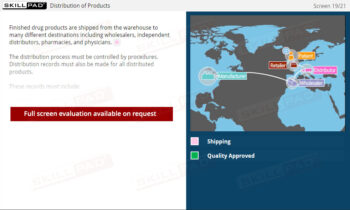
- Learn Label Control and Reconciliation Procedures: Gain practical knowledge of label issuance, storage, and reconciliation processes to minimize errors and uphold Good Manufacturing Practices (GMP).
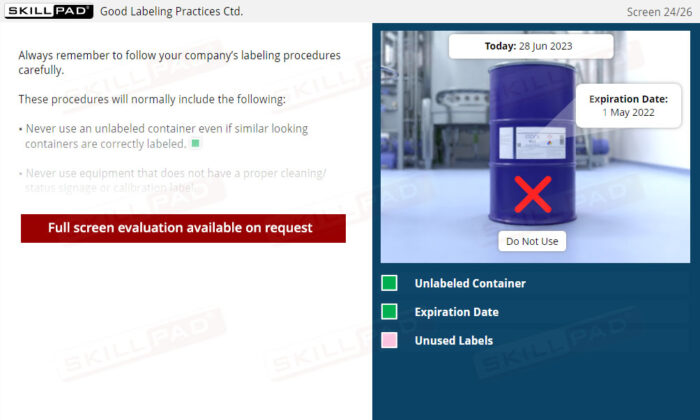
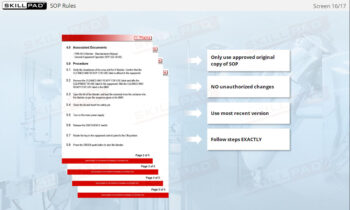
- Follow Good Labeling Practices: Learn best practices for labeling to ensure accuracy and traceability from production to distribution, supporting quality control and regulatory adherence.
Learning Objectives
- State why labeling is important in a pharmaceutical / biologics manufacturing facility.
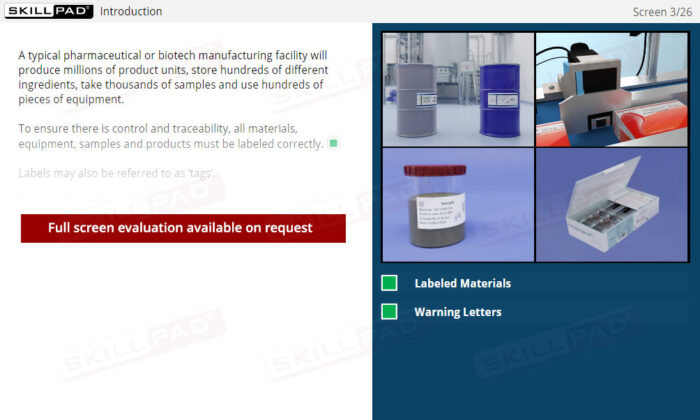
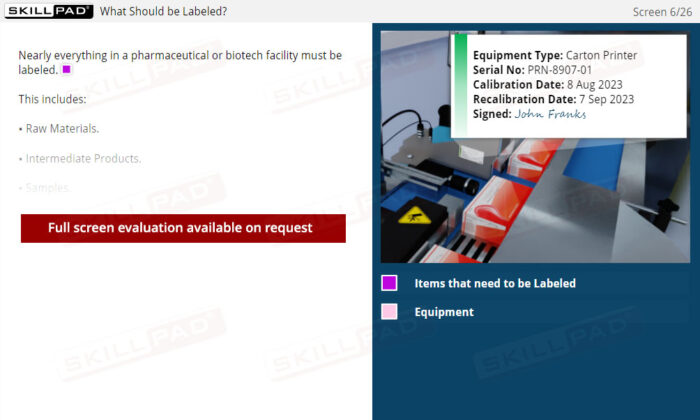
- Identify the items that should be labeled in a pharmaceutical / biologics facility.
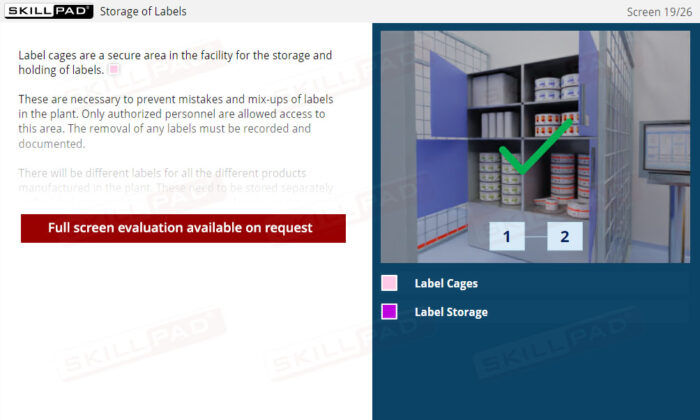
- Explain the term ‘label cage’ and its purpose in a pharmaceutical / biologics facility.
- Recognize the information to be contained on a label.
- List and explain the different types of equipment labels.
- Define ‘reconciliation’ as it relates to labels.
- Provide examples of Good Labeling Practices.
Keywords
- Biotech Manufacturing
- Calibration Labels
- Good Manufacturing Practices (GMP)
- Label Cage
- Labeling Practices
- Product Identification
- Traceability
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen