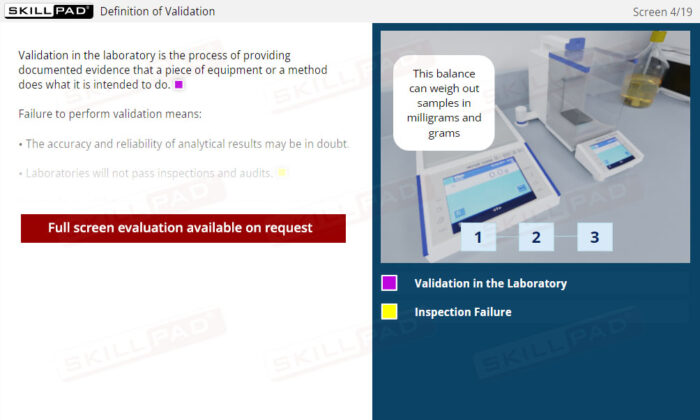
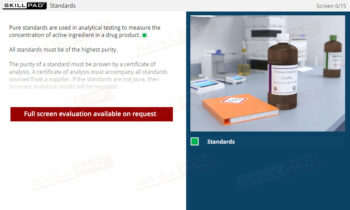
Laboratory Equipment Qualification
Gain a comprehensive understanding of laboratory equipment qualification, focusing on the critical stages that ensure accuracy, reliability, and regulatory compliance. This module explains the foundational concepts of validation and equipment qualification, delving into Design, Installation, Operational, and Performance Qualification. Learn about the importance of calibration, preventative maintenance, and thorough documentation practices required to maintain equipment throughout its operational lifetime.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Solid Understanding of Validation and Qualification: Develop a clear grasp of key concepts, including validation and laboratory equipment qualification, ensuring you can articulate their significance in a regulated environment.
- Insight into Qualification Stages: Familiarize yourself with the four essential stages of laboratory equipment qualification—Design, Installation, Operational, and Performance Qualification—and the distinct requirements of each.
- Practical Knowledge of Documentation: Learn the necessity of rigorous documentation and how to prepare protocols, reports, and evidence to satisfy regulatory inspections and audits.
- Enhanced Awareness of Equipment Maintenance: Understand the role of preventative maintenance, calibration, and requalification in sustaining equipment performance and maintaining compliance with industry standards.
- Regulatory Compliance Mastery: Equip yourself with the knowledge needed to ensure that laboratory instruments adhere to FDA regulations, including the 21 CFR Part 11 guidelines for computer systems and software validation.
Learning Objectives
- Define the terms Validation and Laboratory Equipment Qualification.
- List the four stages of Laboratory Equipment Qualification.
- Describe each of the four stages of Laboratory Equipment Qualification: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
- Explain the terms Calibration and Preventative Maintenance.
Keywords
- Analytical Laboratory
- Calibration
- Compliance
- Design Qualification (DQ)
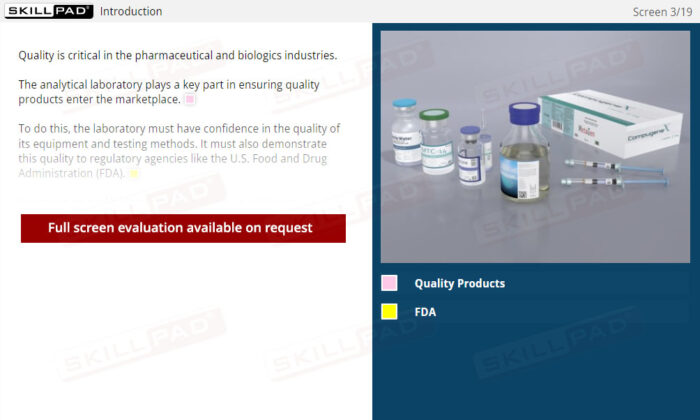
- FDA
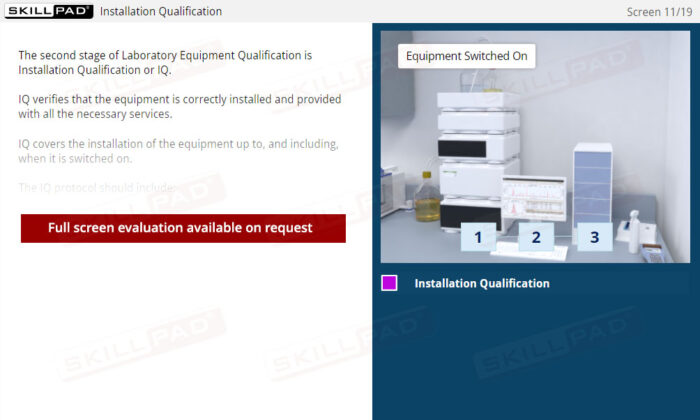
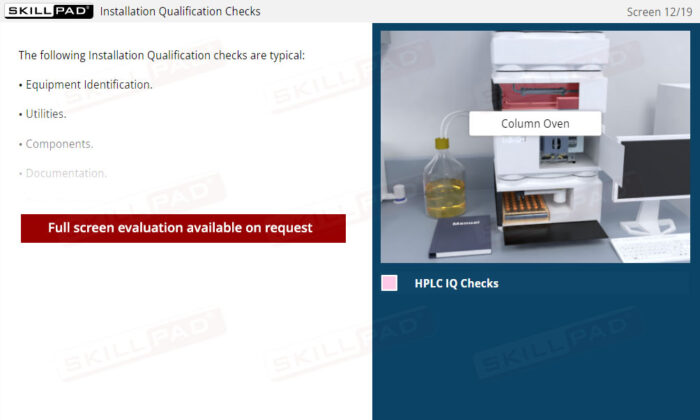
- Installation Qualification (IQ)
- Laboratory Equipment Qualification
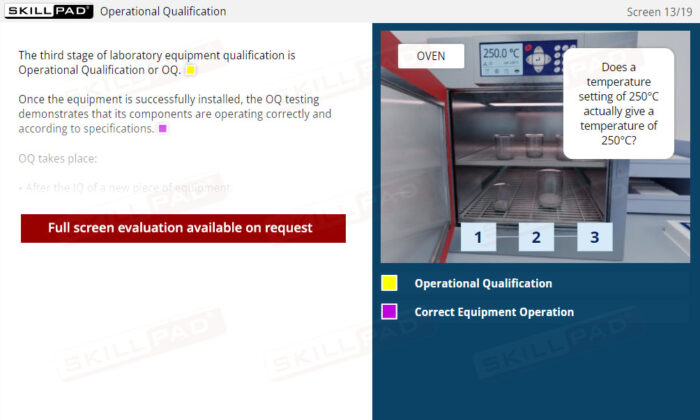
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Quality Assurance
- Validation Process
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen