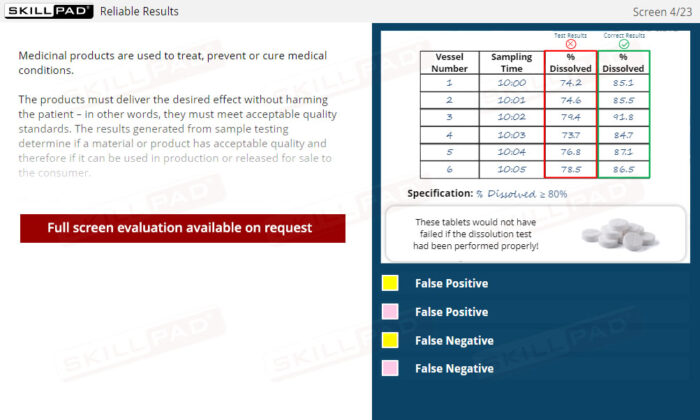
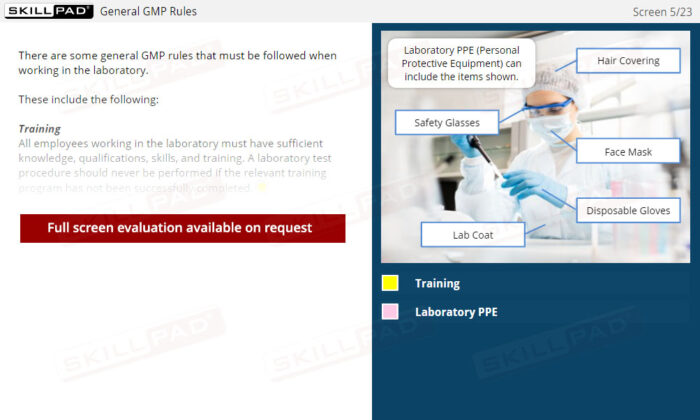
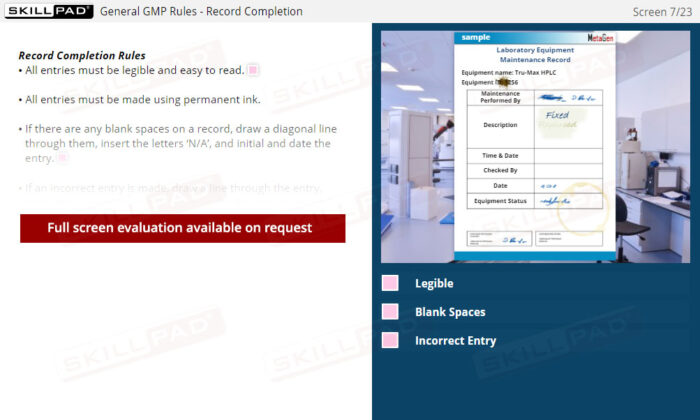
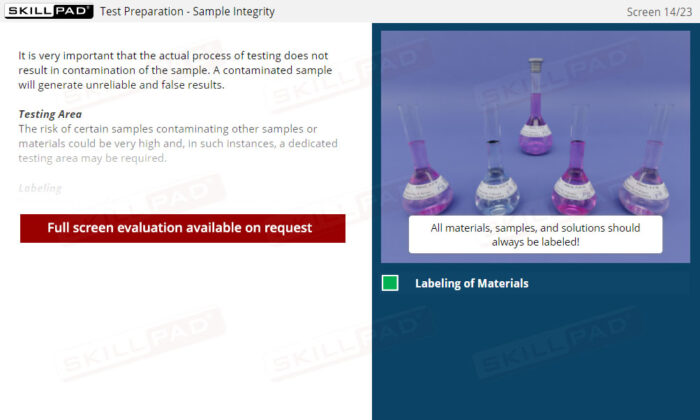
Laboratory GMP
Gain a comprehensive understanding of analytical laboratory Good Manufacturing Practice (GMP) requirements and their significance in maintaining the accuracy and reliability of laboratory test results. This module delves into essential laboratory responsibilities, detailing the procedures analysts must follow, from sample receipt and storage to data handling and result approval. Topics covered include general GMP rules, proper documentation practices, sample testing workflows, solution and equipment preparation, and maintaining compliance with regulatory expectations.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Essential GMP Knowledge for Laboratory Personnel: Understand the fundamental responsibilities of an analytical laboratory and the role of GMP in ensuring accurate, reliable test results.
- Practical Understanding of Laboratory Rules and Compliance: Learn about general GMP rules, the importance of proper training, and behavioral guidelines to prevent contamination and errors in the laboratory.
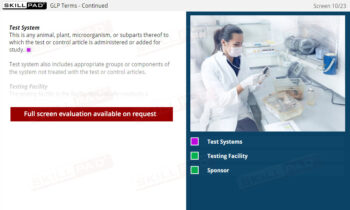
- Mastery of Documentation and Record-Keeping: Develop skills to accurately complete laboratory records, ensuring traceability and compliance with regulatory inspections.
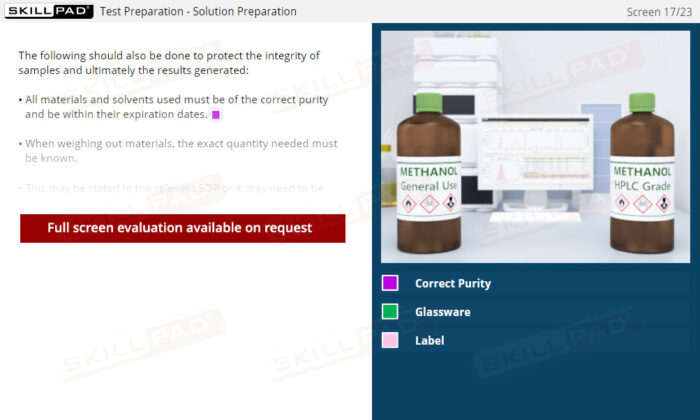
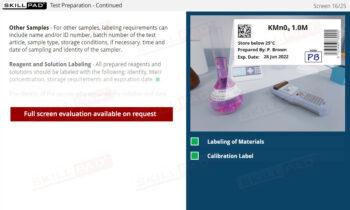
- Proficiency in Sample Handling and Preparation: Grasp the necessary steps for sample receipt, storage, and preparation, emphasizing correct labeling, storage conditions, and segregation requirements to maintain sample integrity.
- Systematic Approach to Testing and Data Management: Familiarize yourself with the procedures for solution and equipment preparation, data generation, and the process of checking and approving results, enhancing overall laboratory efficiency and adherence to GMP standards.
- Awareness of Equipment and Method Compliance: Learn the importance of using calibrated equipment, adhering to system suitability checks, and following SOPs meticulously to produce valid and reliable results.
Learning Objectives
- Explain what the laboratory is responsible for and the purpose of GMP in the laboratory.
- Describe the general GMP rules that apply to the laboratory.
- Complete laboratory records correctly.
- Describe what is involved in sample receipt and storage.
- Outline the steps that the analyst must take to prepare for testing of solution and equipment preparation.
- Describe what is done with data generated through sample analysis.
- Explain how a sample result is released from the laboratory.
Keywords
- Analyst Training
- Analytical Quality Control
- Data Integrity
- Equipment Preparation
- Good Manufacturing Practice
- Laboratory GMP
- Laboratory Record Keeping
- Laboratory Safety
- LIMS (Laboratory Information Management System)
- Regulatory Compliance
- Sample Testing
- Sample Handling
- SOP Compliance
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen