Laboratory Information Management System
Explore the purpose and functionality of Laboratory Information Management Systems (LIMS) used in analytical laboratories. This module delves into the sample management process, from initial receipt and data logging through to quality control, result approval, and batch release. It covers key features such as automated result calculation, controlled access for data integrity, and handling out-of-specification results to maintain compliance and traceability.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Clear Understanding of LIMS Purpose and Functionality: Learn how LIMS systems streamline sample management, from logging samples to automating result reporting and supporting batch release decisions.
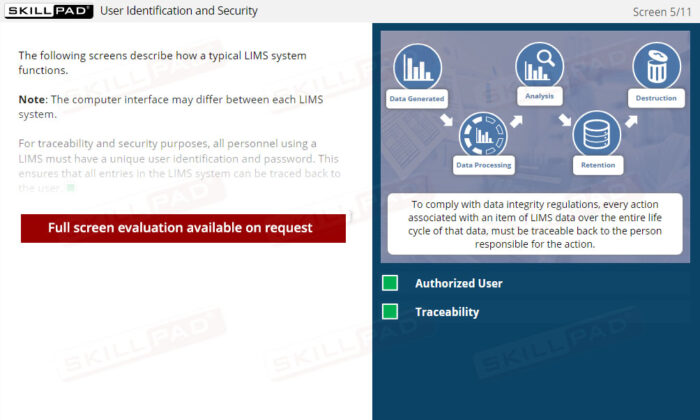
- In-depth Knowledge of Data Management and Security: Understand the importance of controlled access and the role of unique user credentials in ensuring data integrity and traceability within a regulated environment.
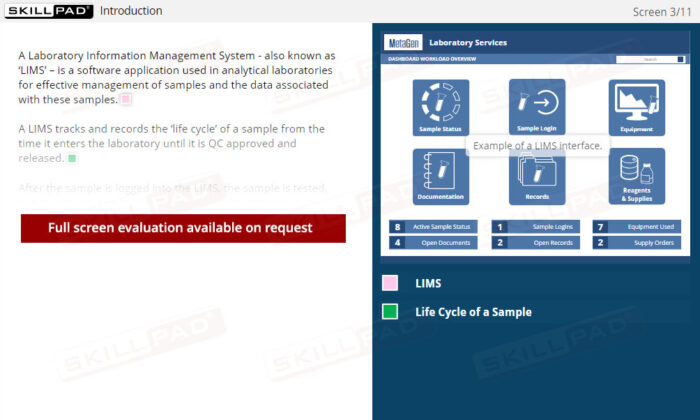
- Comprehensive Overview of Sample Lifecycle: Gain insight into how samples are processed and monitored, including data points like batch numbers, sample status, and processing stages for seamless laboratory operations.
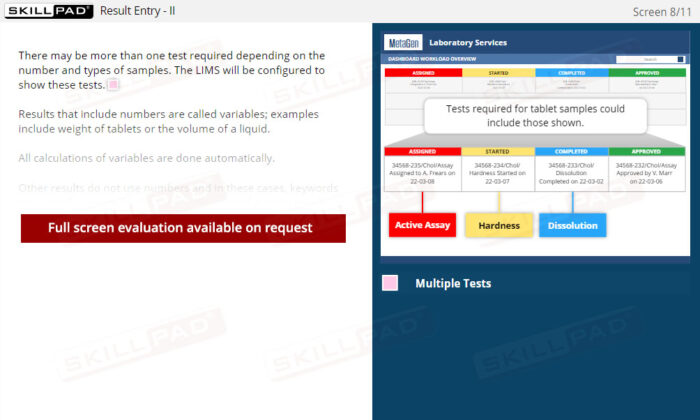
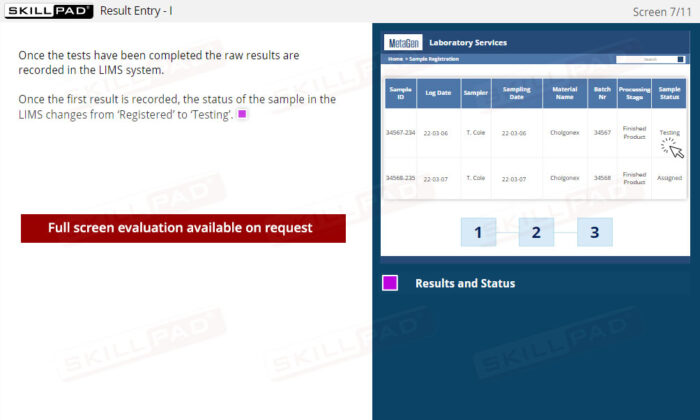
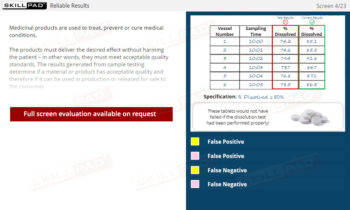
- Effective Handling of Test Results: Master how LIMS manage raw data, automate calculations, and provide visual status indicators for quick identification of in-specification or out-of-specification results.
- Insight into Role-based Access and Authority Levels: Recognize the permissions framework, from analysts performing tests to managers authorizing final batch release, ensuring compliance with regulatory standards.
Learning Objectives
- Define ‘LIMS’ and explain its purpose.
- Describe the main functions of a LIMS.
- Explain why controlled access is essential for LIMS security.
- List examples of data recorded for sample entry in a LIMS.
- Describe how test results are handled by a LIMS including Out-of-Specification (OOS) results.
Keywords
- Analytical Results
- Audit Trails
- Batch Release
- Controlled Access
- Data Integrity
- Data Security
- Laboratory Information Management System (LIMS)
- Out of specification (OOS)
- Quality Control (QC)
- Regulatory Compliance
- Sample Processing
- Sample Tracking
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen