New Drug Development and Clinical Trials
An essential overview of the drug development process, guiding learners through the stages from initial research to regulatory approval. This module covers the intricate path of new drug discovery, nonclinical studies, clinical trials, and regulatory requirements. It emphasizes the critical roles of safety, efficacy, and quality in developing new drugs and provides insight into the regulatory framework designed to protect patient health. Learners will explore each stage of development in depth, from lab-based research to Phase I-IV clinical trials and understand the importance of compliance and data integrity throughout.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the Drug Development Process: Learn the key stages of drug development, from research and nonclinical studies to clinical trials and regulatory approval, to navigate the complexities of the pharmaceutical industry.
- Ensure Drug Safety, Efficacy, and Quality: Explore how regulatory agencies enforce standards to maintain the safety, efficacy, and quality of pharmaceutical products.
- Navigate Regulatory Compliance Requirements: Understand the role of regulatory bodies like the FDA and EMA in drug approval and the compliance requirements for bringing new drugs to market.
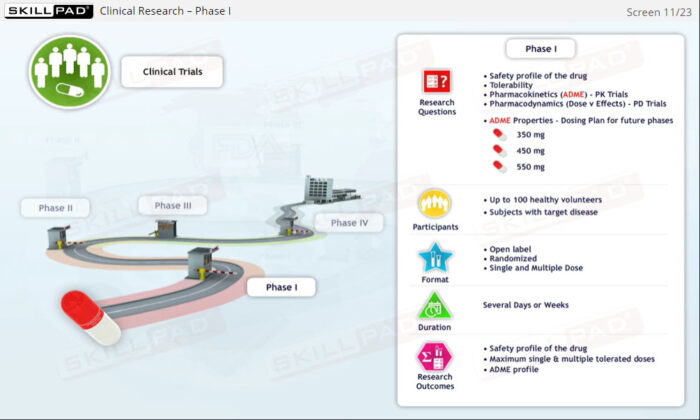
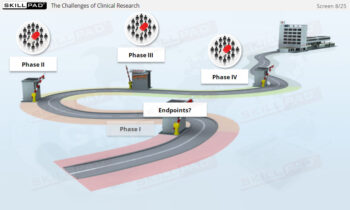
- Explore Clinical Trial Phases: Learn the objectives, methodologies, and significance of Phase I-IV clinical trials in evaluating a drug’s safety and effectiveness.
Learning Objectives
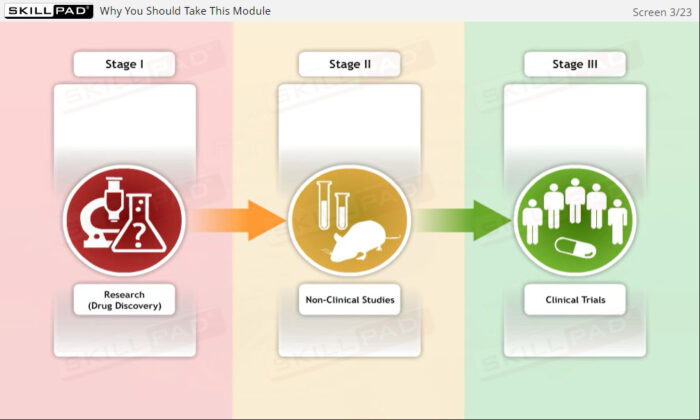
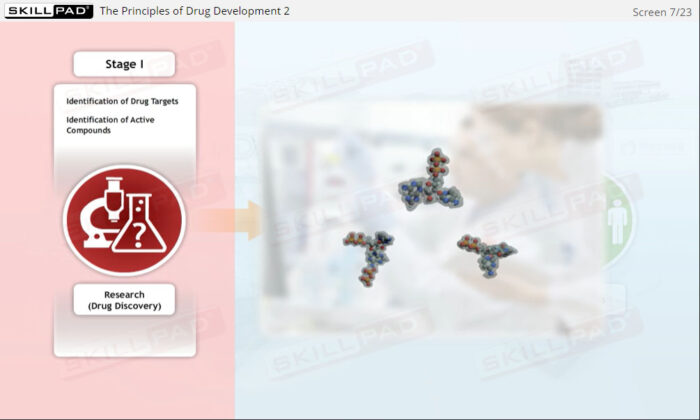
- Give an overview-level explanation of the drug development process and the various phases of clinical trials.
- Explain why the development and testing of new drug products must be regulated.
- Explain the term: Good Clinical Practice (GCP).
- Describe the most important characteristics of a drug product.
Keywords
- ADME (Absorption, Distribution, Metabolism, Excretion)
- Clinical Trials
- Drug Discovery
- EMA
- FDA
- New Drug Development
- Pharmacology
- Toxicology
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen