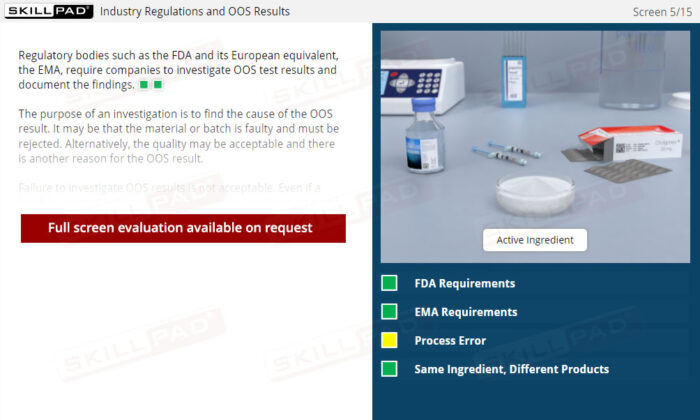
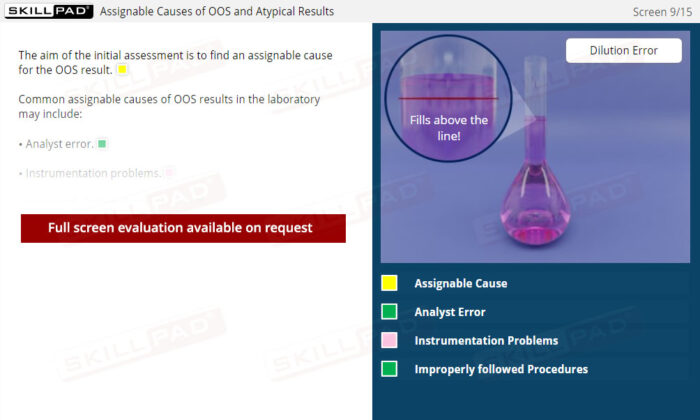
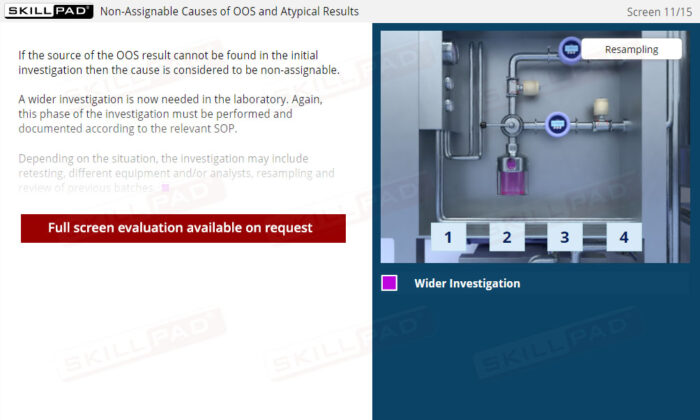
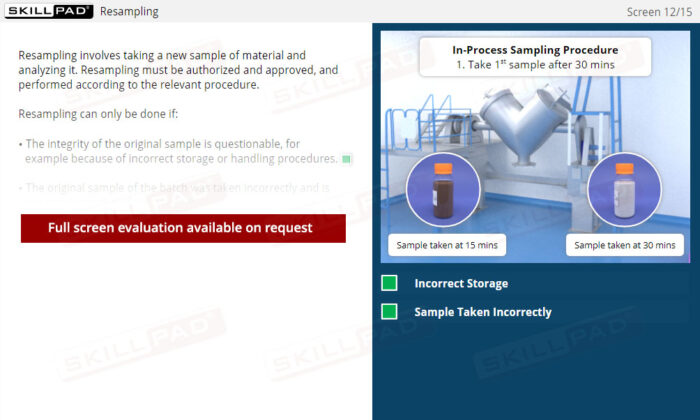
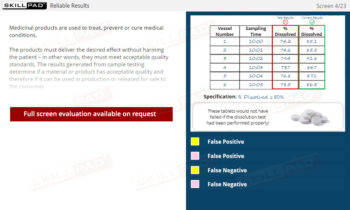
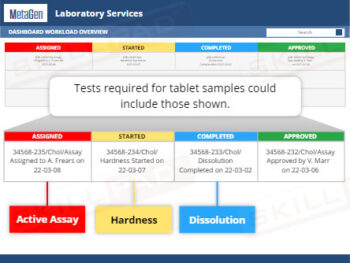
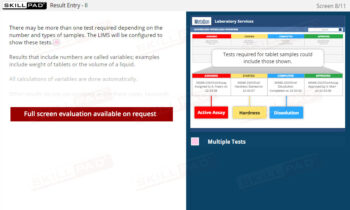
Out of Specification and Atypical Results
An in-depth look at Out of Specification (OOS) and Atypical results in pharmaceutical quality control. It examines the definitions, significance, and causes of these results and the regulatory requirements for investigating them. The content outlines the structured investigation process, starting from laboratory assessment to wider, documented procedures, emphasizing the importance of finding an assignable cause, addressing potential laboratory and process errors, and taking corrective actions. Key regulatory standards, such as the FDA’s Barr Decision, are discussed to highlight industry expectations for scientifically defensible investigations and quality assurance.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Clear Understanding of OOS and Atypical Results: Gain a comprehensive understanding of OOS and Atypical results, their definitions, and why addressing these outcomes is crucial in pharmaceutical quality assurance.
- Knowledge of Regulatory Requirements for Investigations: Learn about regulatory mandates such as the FDA’s Barr Decision, emphasizing the need for thorough documentation and scientifically sound investigations of OOS results.
- Insight into Investigation Processes and Protocols: Acquire step-by-step knowledge of laboratory and production investigations, including initial assessments, identifying assignable causes, retesting protocols, and conditions for resampling.
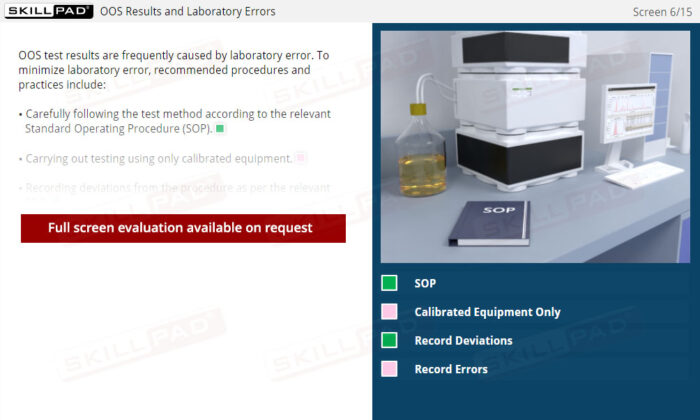
- Skill in Identifying and Mitigating Common Errors: Understand common sources of laboratory errors and process deviations, equipping you with best practices to prevent OOS occurrences, improve testing accuracy, and ensure quality control.
Learning Objectives
- Define the terms ‘Out Of Specification’ (OOS) and ‘Atypical Results’.
- Explain why OOS and Atypical Results are important.
- Describe how these results can occur.
- Describe ways in which OOS and Atypical Results can be prevented.
- Describe the sequence of steps taken to investigate OOS and Atypical Results.
Keywords
- Analytical Testing
- Assignable Cause
- Atypical Results
- Barr Decision
- Investigation Process
- Laboratory Error
- Out of Specification (OOS)
- Process Error
- Quality Control
- Regulatory Compliance
- Standard Operating Procedure (SOP)
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen