Overview of Biopharmaceutical Manufacturing
This Module is aimed at biopharmaceutical manufacturing personnel who require an overview of the main processes and conditions involved in therapeutic protein manufacture including upstream processing, downstream processing as well as formulation and fill finish.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
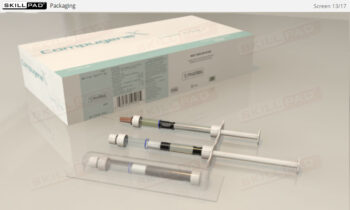
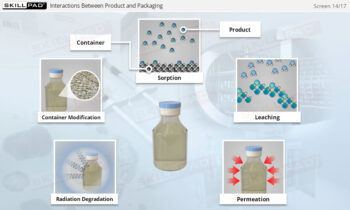
- Gain a thorough understanding of the biopharmaceutical manufacturing process, from the initial cell cultivation to the final product formulation and packaging.
- Enhance your knowledge of relevant Good Manufacturing Practices (GMP), ensuring adherence to industry standards and regulatory requirements for biopharmaceutical production.
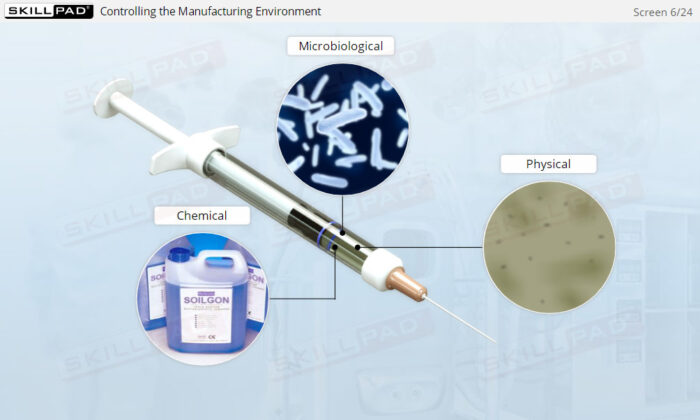
- Understand the importance of aseptic processing, cleanrooms, and the critical measures needed to control microbial contamination throughout the manufacturing process.
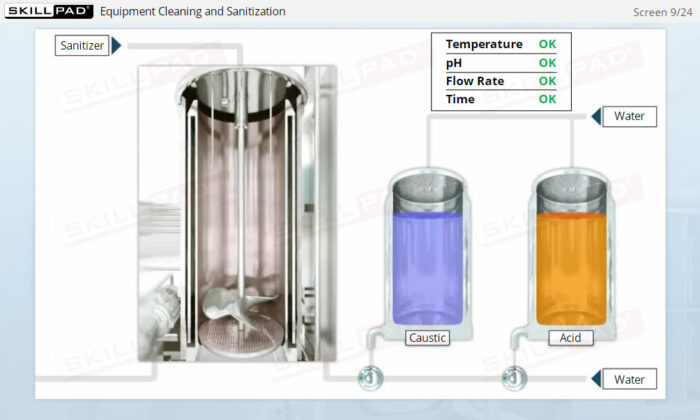
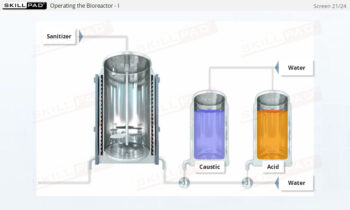
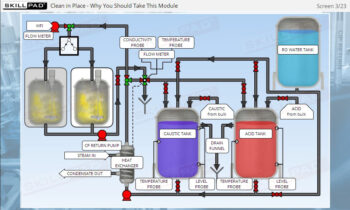
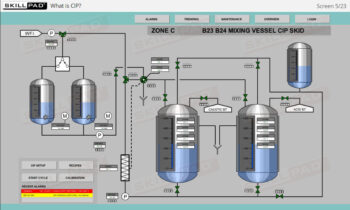
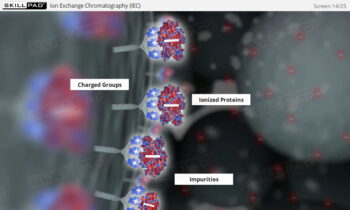
- Develop a strong grasp of upstream and downstream processing stages, including key contamination control measures such as CIP (Clean-In-Place) and SIP (Steam-In-Place).
Learning Objectives
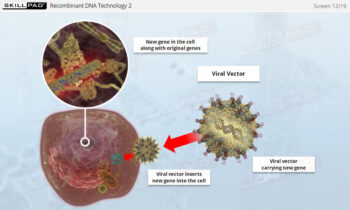
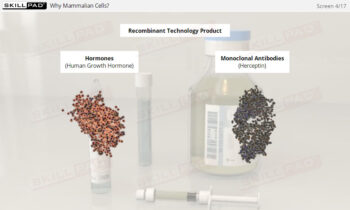
- Describe the main types of biopharmaceutical products.
- Explain why following GMP is critical in biopharmaceutical manufacturing.
- Explain why aseptic processing and clean rooms are critical in biopharmaceutical manufacturing.
- Explain the terms CIP and SIP and their importance in a biopharmaceutical manufacturing process.
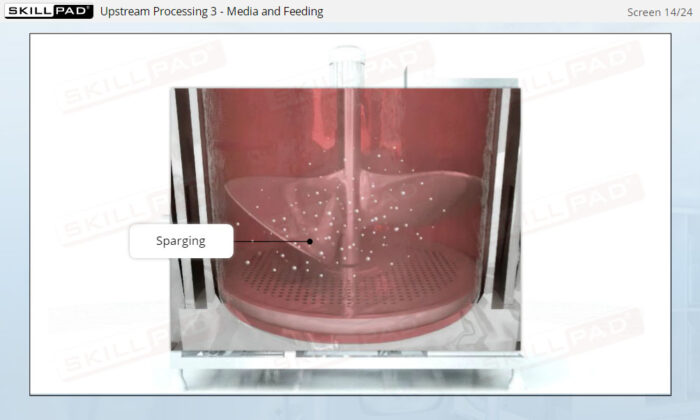
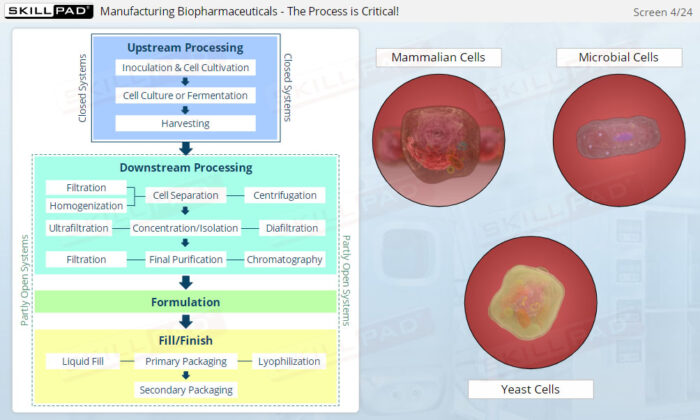
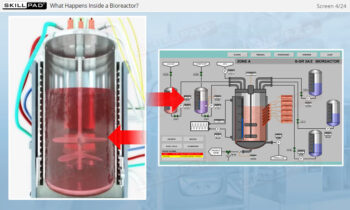
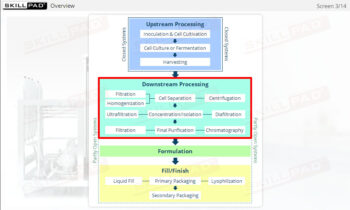
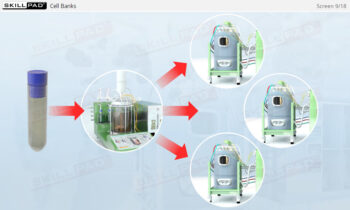
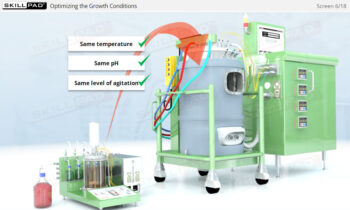
- Describe the different stages of upstream processing.
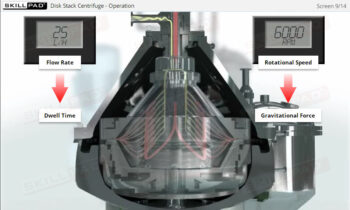
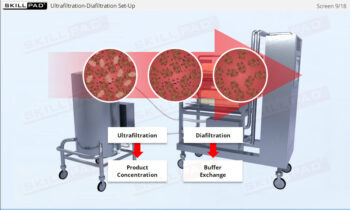
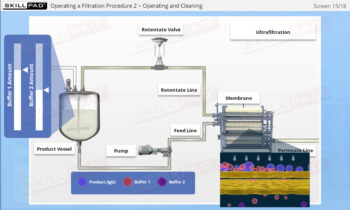
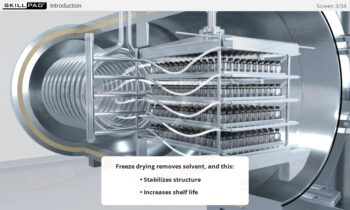
- Describe the different stages of downstream processing.
- Explain the purpose of product formulation.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen