Overview of GxP
The essential principles and regulations of Good Laboratory Practice (GLP), Good Clinical Practice (GCP), and Good Manufacturing Practice (GMP), collectively referred to as GxP. This module outlines the importance of these standards in safeguarding drug safety, efficacy, and quality throughout the drug development lifecycle, from research and nonclinical studies to clinical trials and manufacturing. It explores the role of regulatory bodies, such as the FDA and EMA, and highlights the critical consequences of non-compliance, reinforcing the significance of maintaining rigorous compliance in every phase of pharmaceutical production.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand GxP Regulations and Their Role: Learn the fundamentals of GLP, GCP, and GMP and their specific roles in the research, development, and manufacture of medicinal products to ensure their safety, efficacy, and quality while protecting the patients who rely on them.
- Navigate Drug Development and Approval Processes: Explore the journey of a drug from initial research to post-market surveillance, including key regulatory milestones enforced by agencies like the FDA and EMA.
- Recognize the Impact of Regulatory Non-Compliance: Understand the financial, legal, and reputational consequences of non-compliance and how adherence to GxP standards supports organizational integrity.
- Gain Insights into Compliance Best Practices: Learn how to support your organization’s compliance efforts by understanding and implementing industry best practices.
Learning Objectives
- List the three characteristics that all drug products must possess.
- Describe the drug development and approval process.
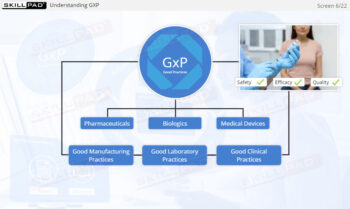
- Define GxP and why it is necessary.
- Explain GLP, GCP, and GMP in terms of their specific objectives.
- Describe the functions of regulatory agencies such as the FDA and EMA.
- Describe the consequences of non-compliance with GxPs.
Keywords
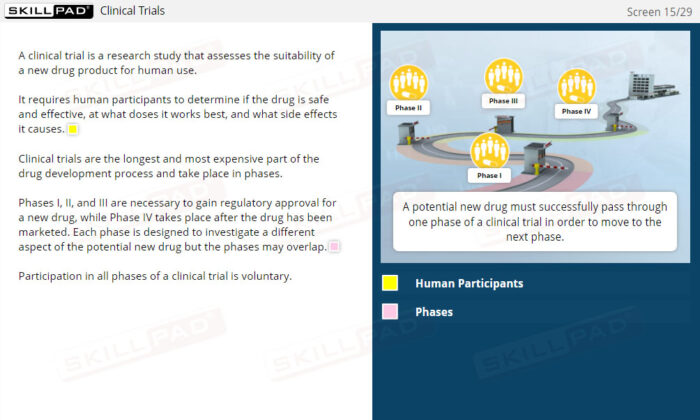
- Clinical Trials
- Drug Development Process
- Drug Efficacy
- Drug Quality
- Drug Safety
- European Medicines Agency (EMA)
- Food and Drug Administration (FDA)
- Good Clinical Practice (GCP)
- Good Laboratory Practice (GLP)
- Good Manufacturing Practice (GMP)
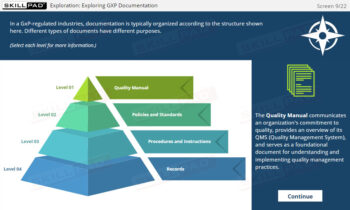
- GxP Regulations
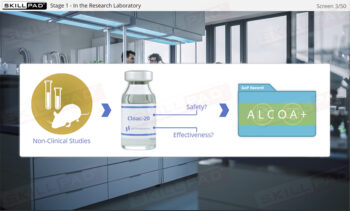
- Nonclinical Studies
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen