Personnel and Training
An introduction to the roles, responsibilities, and regulatory requirements of personnel in pharmaceutical manufacturing environments. The module explains how GMP regulations apply to personnel, highlights the importance of having qualified staff, and describes the organizational structure of manufacturing facilities. It outlines key personal responsibilities, including training, clothing, hygiene, and health, and explains how regulatory bodies assess training practices during inspections.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
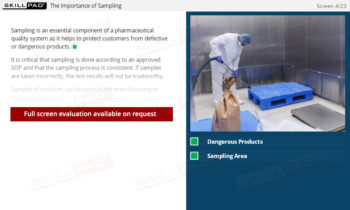
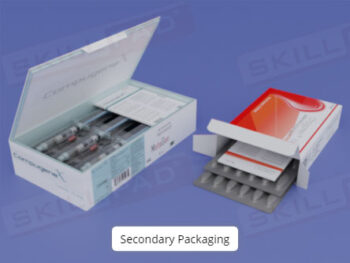
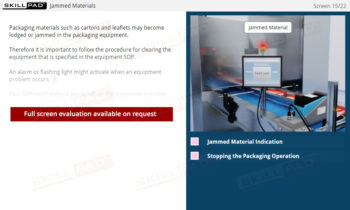
- Learn GMP Regulations for Personnel: Understand key GMP requirements for personnel qualifications, training, clothing, hygiene, and health, and their importance for product safety and regulatory compliance.
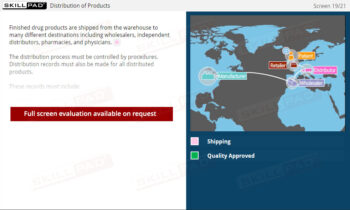
- Understand Facility Organization: Learn how pharmaceutical facilities are structured, key departmental roles, and how a well-organized facility prevents errors and ensures product quality.
- Clarify Responsibilities and Expectations: Identify personal responsibilities, such as following GMP, using protective equipment correctly, and maintaining hygiene to prevent contamination.
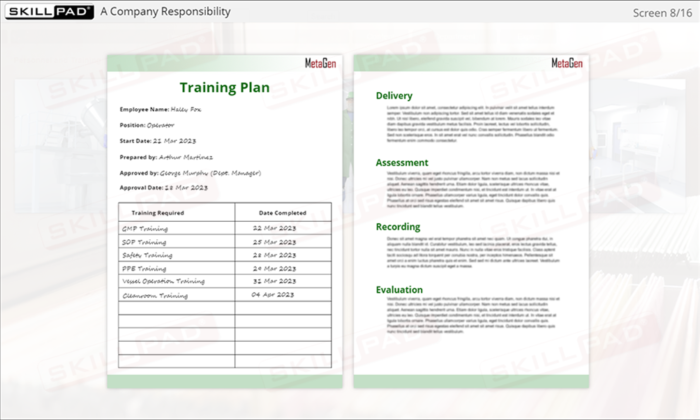
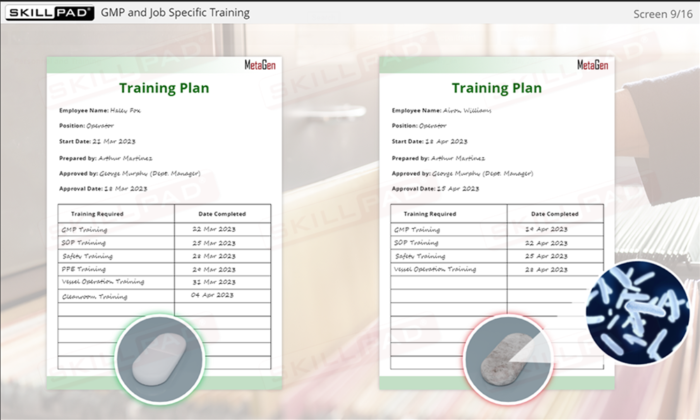
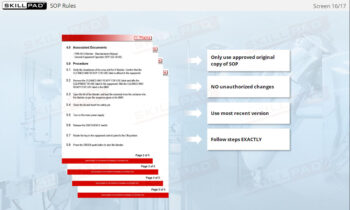
- Learn Effective Training Practices: Differentiate between GMP and job-specific training, understand training plans, and how training records are maintained and assessed for compliance.
- Prepare for Regulatory Assessments: Understand what inspectors review in training records and systems and how to demonstrate compliance during audits.
- Commitment to GMP Standards: Recognize how adhering to GMP practices supports product safety and quality, emphasizing clear communication and procedural compliance.
Learning Objectives
- List areas of a pharmaceutical manufacturing operation where GMP regulations apply.
- Explain why ‘qualified’ personnel are essential in a pharmaceutical company.
- Describe how a pharmaceutical manufacturing facility is typically organized in terms of departments.
- List examples of areas of personal responsibility within a pharmaceutical manufacturing facility.
- Distinguish between GMP and Job Specific training.
- Describe the typical approach to training by regulatory agencies during an inspection of a pharmaceutical facility.
Keywords
- Pharmaceutical Training
- Contamination Prevention
- Employee Commitment
- Facility Inspections
- FDA Inspections
- GMP Training
- Job-Specific Training
- Personal Hygiene
- Personal Protective Equipment (PPE)
- Personnel Qualifications
- Pharmaceutical Manufacturing
- Pharmaceutical Personnel
- Regulatory Compliance
- SOP Adherence
- Training Records
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen