Primary Packaging
An overview of primary packaging processes in pharmaceutical manufacturing, covering essential components such as container types, contamination prevention, and in-process checks. This module provides a foundational understanding of the setup and execution of primary packaging operations, including critical procedures like equipment calibration, labeling, filling, and sealing within controlled environments. The importance of integrity tests, reconciliation practices, and cleaning protocols is emphasized to ensure safety, quality, and regulatory compliance.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
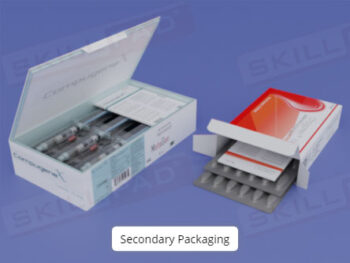
- Learn About Primary Packaging: Understand the purpose and importance of primary packaging and the differences between primary, secondary, and tertiary packaging in pharmaceutical production.
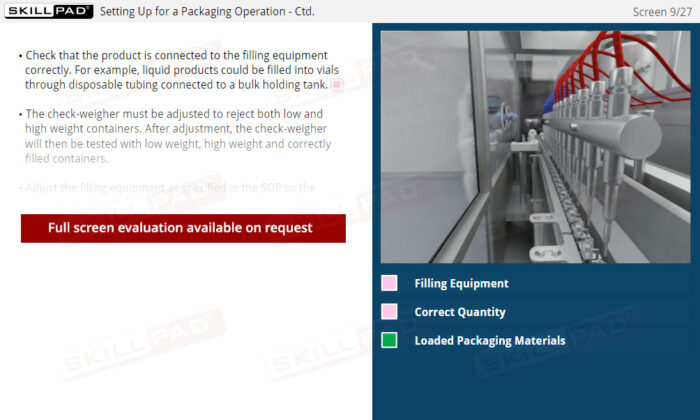
- Understand Packaging Equipment and Setup: Gain foundational knowledge of equipment checks, calibration, and setup requirements to support safe and effective packaging operations.
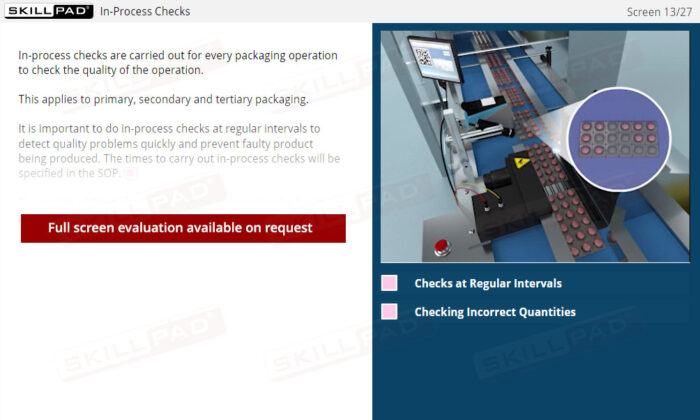
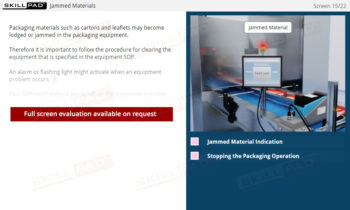
- Perform In-Process Quality Checks: Learn how to conduct essential in-process checks, perform integrity tests, and detect and address defects to maintain product quality and ensure regulatory compliance.
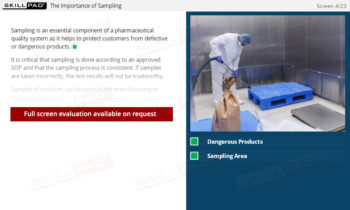
- Learn Contamination Control Methods: Explore ways to maintain a controlled environment to protect products from contamination during the packaging process.
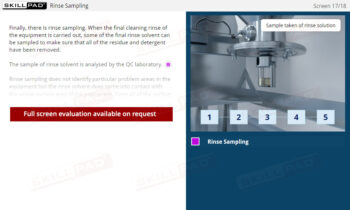
- Understand Reconciliation and Cleaning Protocols: Learn how to carry out reconciliation to ensure all packaging materials are accounted for and implement cleaning protocols to prevent cross-contamination.
Learning Objectives
- Define the term ‘primary packaging’.
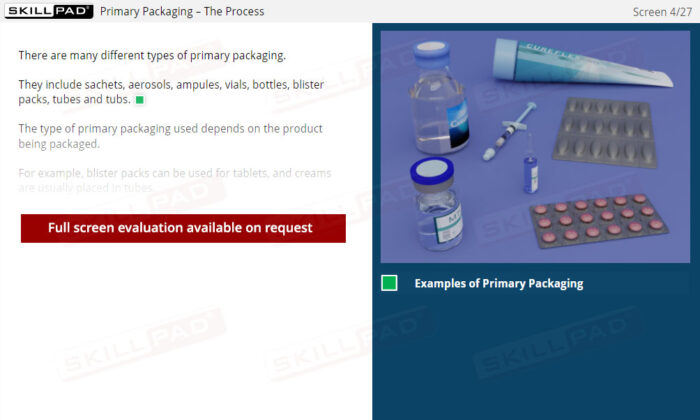
- Name the different types of primary packaging.
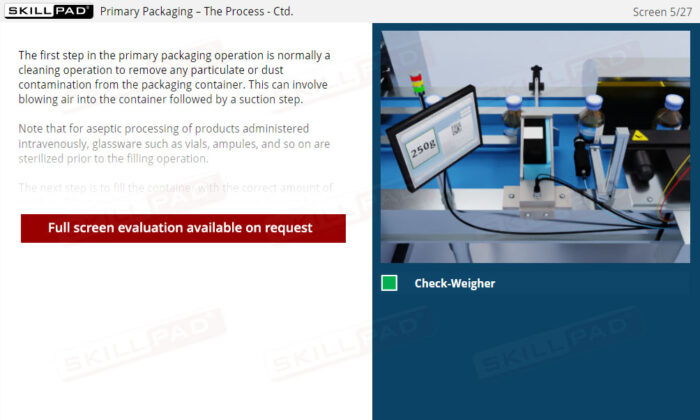
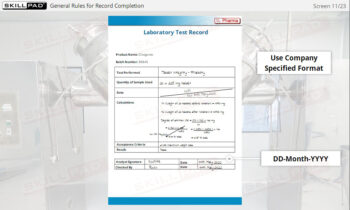
- Describe the checks that have to be made and recorded before starting a primary packaging operation.
- List the in-process checks that are carried out during primary packaging operations.
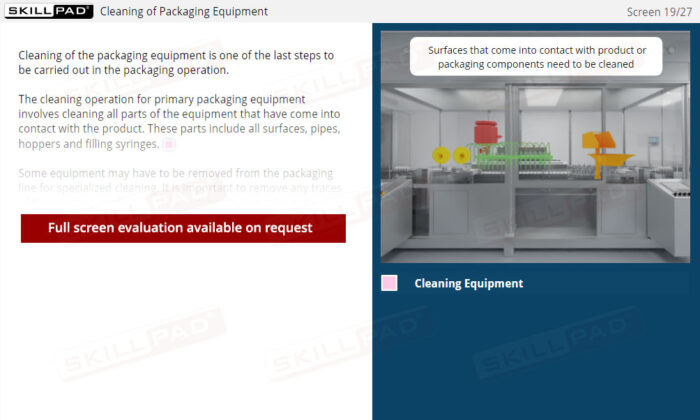
- Explain the cleaning process for primary packaging equipment.
- Define the term ‘reconciliation’.
- Name the items that have to be reconciled.
Keywords
- Aseptic Filling
- Batch Record
- Biologics
- Calibration
- Contamination Prevention
- Controlled Environment
- Filling Operations
- Integrity Tests
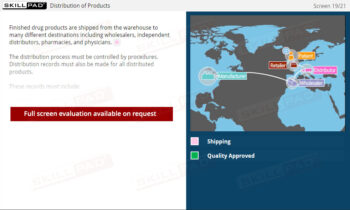
- Primary Packaging
- Reconciliation
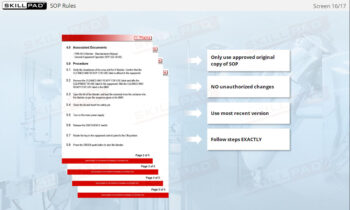
- Standard Operating Procedure (SOP)
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen