Process Validation: Process Design
An overview of the process design stage of process validation, describing how a biopharmaceutical manufacturing process can be defined using a Quality by Design (QbD) approach that emphasizes accumulated scientific knowledge and quality risk management.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
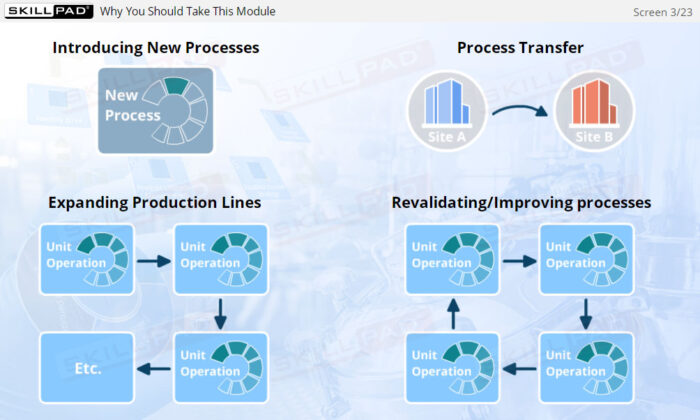
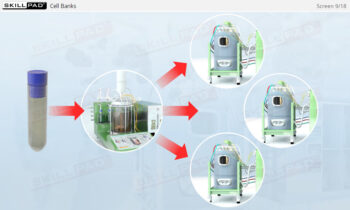
- Gain a thorough understanding of Process Design as part of Process Validation, focusing on building quality into biopharmaceutical manufacturing processes from the beginning.
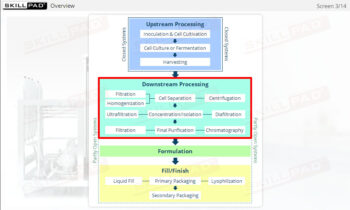
- Learn about the three stages of Process Validation, emphasizing the importance of Process Design in ensuring products consistently meet quality specifications.
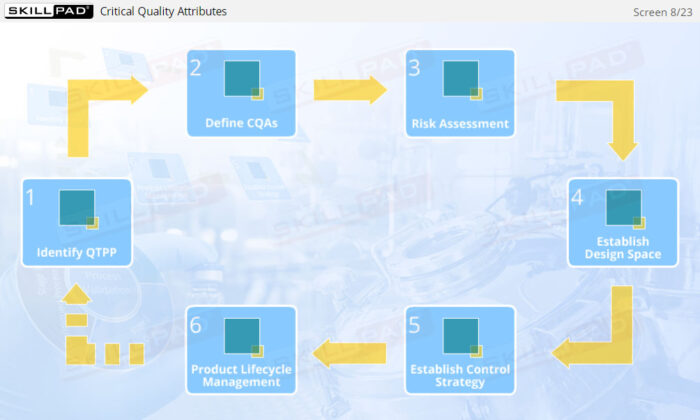
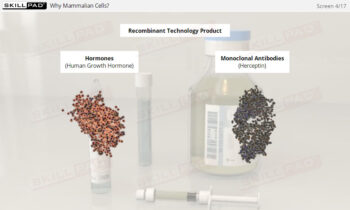
- Enhance your knowledge of essential quality management concepts, including Quality by Design (QbD), Quality Target Product Profile (QTPP), and Critical Quality Attributes (CQA).
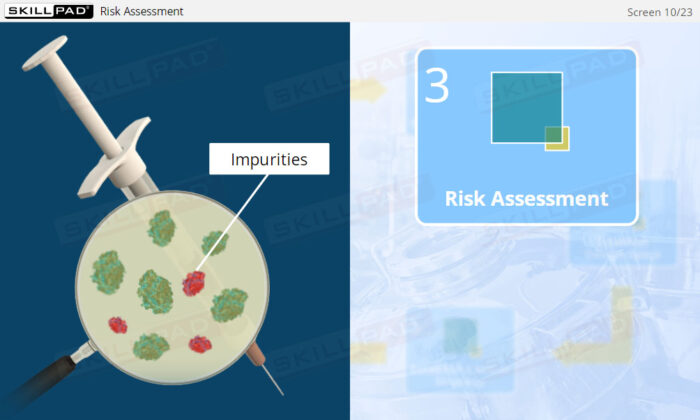
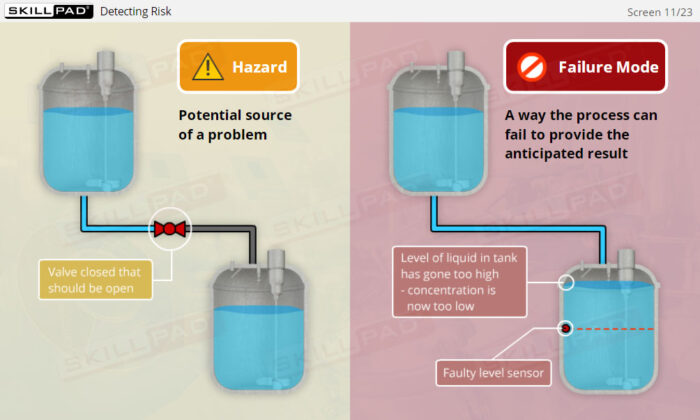
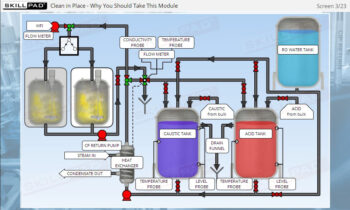
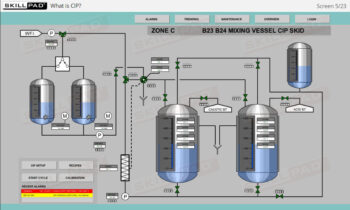
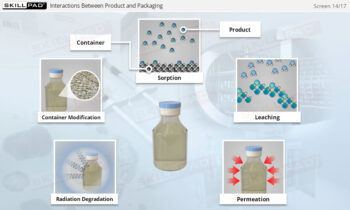
- Learn how risk assessments are conducted to identify and control potential risks that may impact product quality.
- Explore risk assessment tools such as Failure Modes and Effects Analysis (FMEA) and Hazard Analysis and Critical Control Points (HACCP) to effectively manage and mitigate risks.
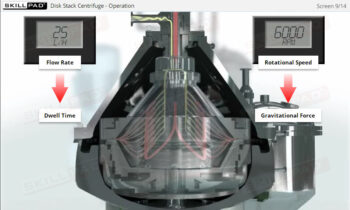
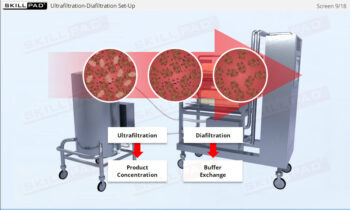
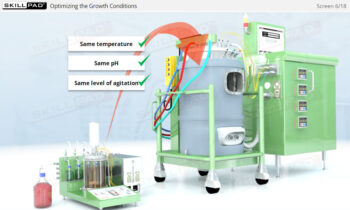
- Discover how a design space is established through Design of Experiments (DOE) to optimize process parameters and ensure consistent product quality.
- Understand the role of lifecycle management in continuously improving process capability and product quality.
Learning Objectives
- Describe the rationale for having a structured approach to Process Design.
- Explain what is meant by ‘Quality by Design’.
- Explain the concept of a ‘Quality Target Product Profile’.
- Define Critical Quality Attribute (CQA) and provide some examples.
- Explain why risk assessment is done as part of process design.
- Explain the concept of manufacturing ‘design space’ with reference to Design of Experiments and Critical Process Parameters.
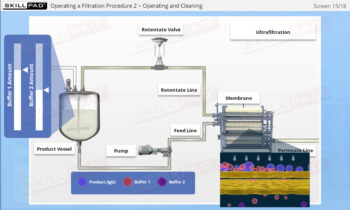
- Define Process Control Strategy and Operational Control Strategy.
- Define Master Production Record and explain its relationship to Process Design studies.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen