Records in API Manufacturing
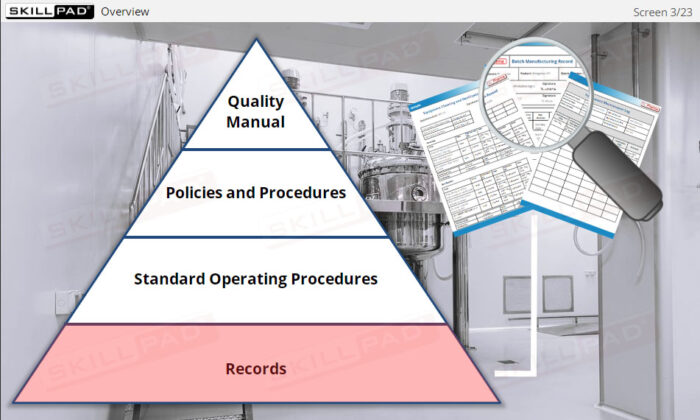
Effective record-keeping is a critical aspect of API manufacturing operations. This module introduces the essential records used in the manufacturing process, such as production, materials, laboratory, and distribution records. It emphasizes the importance of traceability, proper completion, and retention of records to ensure GMP compliance. Learners will explore common types of records, their content, and the rules for accurate documentation, enabling them to understand the role of records in maintaining product quality and ensuring regulatory compliance.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the Purpose and Importance of Manufacturing Records: Gain comprehensive knowledge of the different types of records required in API manufacturing, including production, materials, laboratory, and maintenance records.
- Build Enhanced Awareness of Traceability: Learn why maintaining accurate records is crucial for traceability in the production process, ensuring the ability to trace any issues back to their source.
- Gain Critical Insights into Record Completion: Learn the rules for completing records, whether paper-based or electronic, including signature requirements, proper notation of errors, and retention timelines.
- Knowledge of Regulatory Compliance: Develop a clear understanding of the importance of accurate documentation to meet GMP standards and the regulatory expectations for maintaining proper records throughout the manufacturing lifecycle.
- Practical Guidance for Daily Operations: Equip yourself with the tools and techniques to complete records correctly and responsibly, ensuring compliance and product quality in every stage of production.
Learning Objectives
- Define ‘record’ as it relates to API manufacturing.
- Explain why records are necessary in API manufacturing.
- List examples of the types of records used in API manufacturing.
- Explain why traceability is essential in API manufacturing.
- Give examples of general rules for completing records.
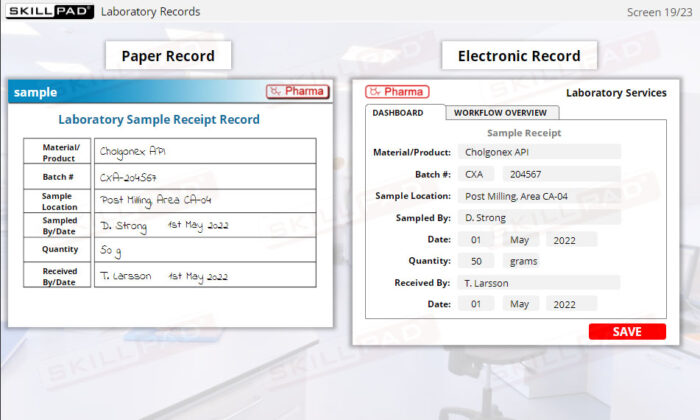
- List the information typically contained in production, materials, laboratory, and distribution records.
- List the information typically contained in equipment maintenance and cleaning records
Keywords
- API Manufacturing
- Batch Production Records
- Cleaning Records
- Documentation System
- Equipment Maintenance
- Equipment Sanitization
- GMP Compliance
- Laboratory Records
- Materials Records
- Product Traceability
- Production Records
- Quality Control
- Record Completion
- Record Retention
- Record Rules
- Regulatory Compliance
- Sanitization
- Traceability
- Training Module
- Validation Records
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen