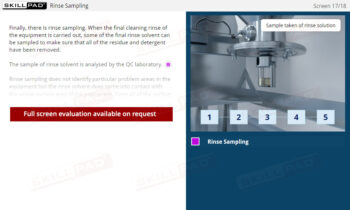
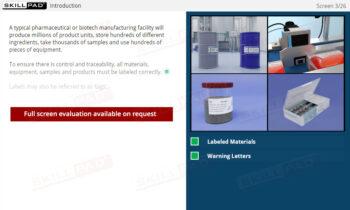
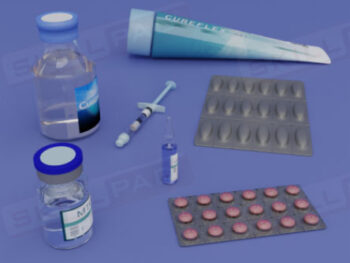
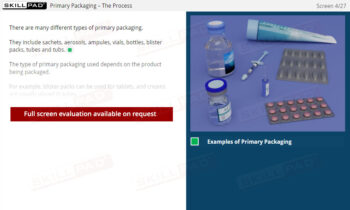
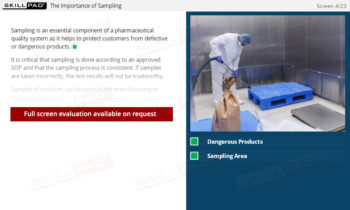
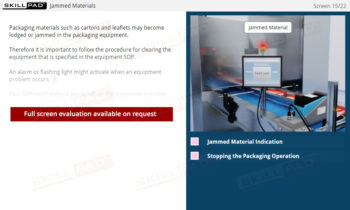
Records in Finished Dose Manufacturing
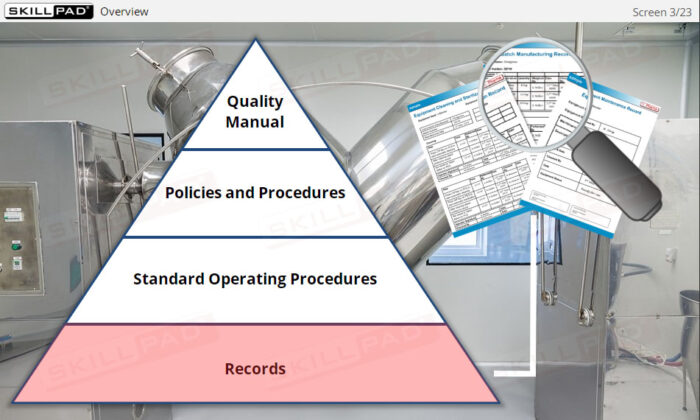
An overview of the essential role of records in finished dose manufacturing, focusing on their purpose, types, and importance in ensuring compliance with Good Manufacturing Practices (GMP). This module explains how records support traceability and provide written evidence that processes have been carried out correctly. Learners will explore key records related to production, materials, equipment use, cleaning, maintenance, and laboratory testing, as well as general rules for completing records accurately to avoid compliance issues and maintain product quality.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the Purpose of Records: Learn why records are vital in finished dose production, providing essential documentation for GMP compliance and product traceability.
- Learn About Different Types of Records: Understand records used in pharmaceutical operations, including production, materials, laboratory, equipment, and distribution records.
- Understand the Importance of Traceability: Learn how records enable product traceability from manufacturing to distribution, critical for identifying and addressing issues.
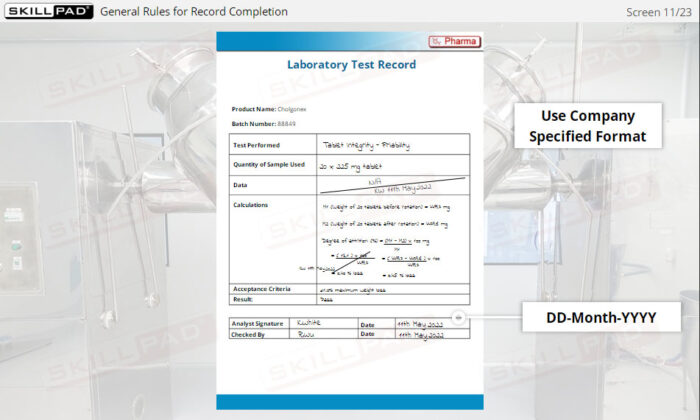
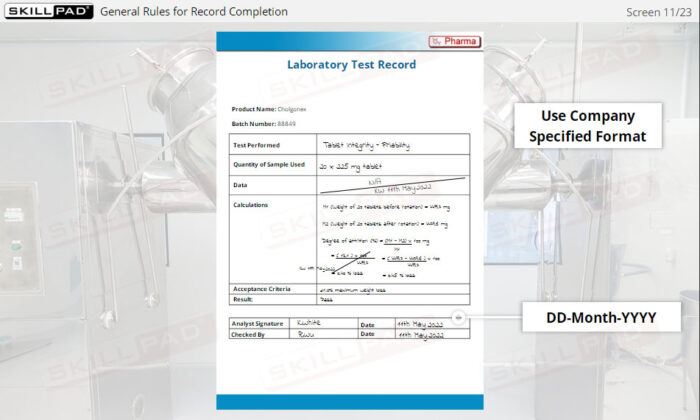
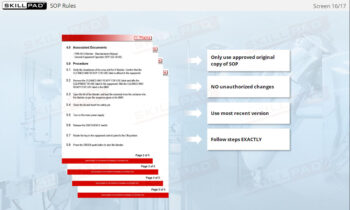
- Follow Best Practices for Record Completion: Learn general rules for accurate record completion, such as using permanent ink, avoiding fluid corrections, and following company-specific formats.
- Recognize Key Record Information: Understand critical details recorded in documents, including batch numbers, materials used, maintenance logs, and laboratory test results.
- Maintain GMP Compliance: Gain confidence in completing records properly, understanding how accurate documentation supports inspections and ensures safe and compliant production of pharmaceuticals.
Learning Objectives
- Explain why records are essential in finished dose manufacturing.
- List examples of the types of records used in finished dose manufacturing.
- Explain why traceability is essential in finished dose manufacturing.
- Define ‘record’ as it relates to finished dose manufacturing.
- Give examples of general rules for completing record.
- List the information typically contained in production, materials, laboratory, and distribution records.
- List the information typically contained in equipment use, maintenance, and cleaning/sterilization records.
Keywords
- Records Management
- GMP Compliance
- Batch Records
- Documentation Accuracy
- Traceability
- Production Records
- Equipment Logs
- Laboratory Records
- Product Distribution
- Record Retention
- Quality Assurance
- Regulatory Compliance
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen