Regulation of the Pharmaceutical Industry
Explore how the pharmaceutical industry is regulated across major global drug markets, focusing on key regulatory authorities, the processes for new drug approval in the US and EU, and the various types of FDA inspections. The module explains the critical roles of regulatory bodies in ensuring compliance with Good Manufacturing Practices (GMP), outlines the consequences of non-compliance, and emphasizes the importance of individual employee responsibility during regulatory inspections.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the Importance of Industry Regulation: Learn why the pharmaceutical industry is highly regulated and how major global regulatory bodies ensure public safety.
- Navigate the Drug Approval Process: Explore the steps and requirements for obtaining drug approval from the FDA and EMA, including essential documentation and market entry criteria.
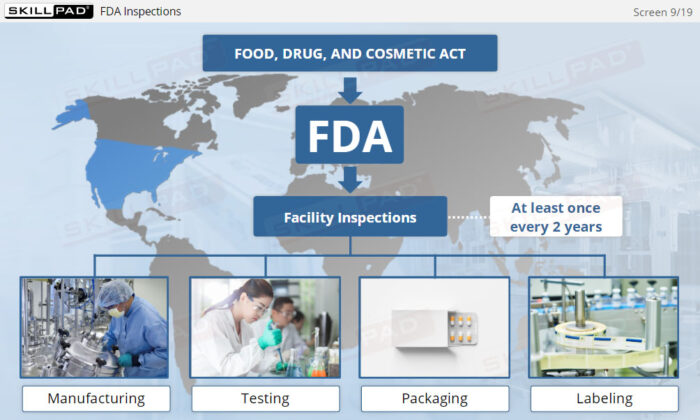
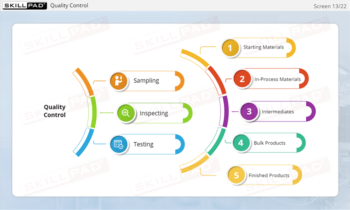
- Recognize the Role of FDA Inspections: Understand the differences between pre-approval, post-approval, and GMP systems inspections and how they ensure ongoing compliance with regulatory standards.
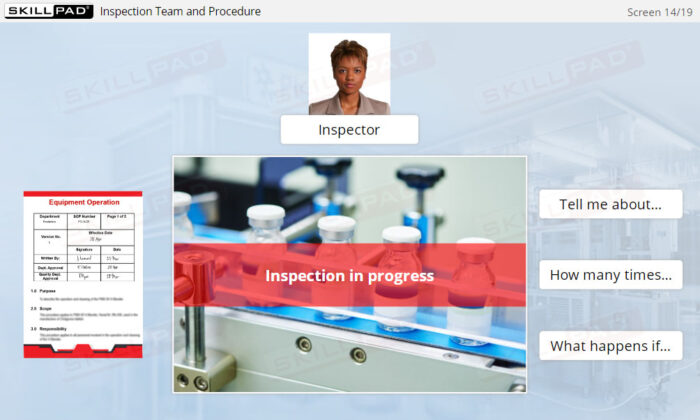
- Prepare for Regulatory Inspections: Learn how employees should conduct themselves during FDA inspections and how GMP compliance impacts the entire organization.
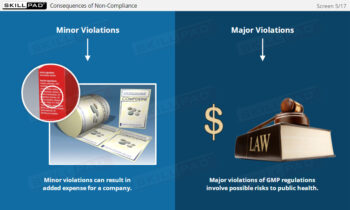
- Understand Inspection Outcomes and Compliance Risks: Explore the implications of FDA 483 reports, Warning Letters, and the consequences of non-compliance, including market bans and product recalls.
Learning Objectives
- Explain why the pharmaceutical industry is regulated.
- List the main regulatory bodies within the global pharmaceutical industry.
- Define the responsibilities of regulatory authorities.
- Explain how the new drug approval process works in both the US and the EU.
- List the three main types of FDA inspection.
- Describe the purpose of each type of FDA inspection.
- Explain the difference between a 483 report and a Warning Letter.
- List three potential consequences of failing to correct violations detailed in an FDA Warning Letter.
Keywords
- 483 Report
- Compliance
- European Medicines Agency (EMA)
- Food and Drug Administration (FDA)
- Good Manufacturing Practices (GMP)
- GMP Systems
- Ministry of Health, Labor, and Welfare (MHLW)
- Pharmaceutical Regulation
- Warning Letter
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen